Scientists use nanoscale catalysts to produce biofuels from syngas
 Researchers at the U.S. Department of Energy’s Ames Laboratory and Iowa State University are combining gasification with high-tech nanoscale porous catalysts, to create ethanol from a wide range of biomass, including distiller’s grain left over from ethanol production, corn stover from the field, grass, wood pulp, animal waste, or garbage.
Researchers at the U.S. Department of Energy’s Ames Laboratory and Iowa State University are combining gasification with high-tech nanoscale porous catalysts, to create ethanol from a wide range of biomass, including distiller’s grain left over from ethanol production, corn stover from the field, grass, wood pulp, animal waste, or garbage.Gasification is a process that turns carbon-based feedstocks under high temperature and pressure in an oxygen-controlled atmosphere into synthesis gas, or syngas. Syngas is made up primarily of carbon monoxide and hydrogen (more than 85 percent by volume) and smaller quantities of carbon dioxide and methane.
It’s basically the same technique that was used to extract the gas from coal that fueled gas light fixtures prior to the advent of the electric light bulb. The advantage of gasification compared to fermentation technologies is that it can be used in a variety of applications, including process heat, electric power generation, and synthesis of commodity chemicals and fuels. Moreover, gasification can turn any biomass feedstock into syngas.
There was some interest in converting syngas into ethanol during the first oil crisis back in the 70s. The problem was that catalysis technology at that time didn’t allow selectivity in the byproducts. They could produce ethanol, but you’d also get methane, aldehydes and a number of other undesirable products. - Victor Lin, Ames Lab Chemical and Biological Science Program DirectorA catalyst is a material that facilitates and speeds up a chemical reaction without chemically changing the catalyst itself. In studying the chemical reactions in syngas conversion, Lin found that the carbon monoxide molecules that yielded ethanol could be “activated” in the presence of a catalyst with a unique structural feature.
If this ‘activated’ CO adsorption on the surface of the catalyst is increased, it improves the opportunity for the formation of ethanol molecules. And if the amount of surface area for the catalyst is increased, the researchers can boost the amount of ethanol produced.
Lin’s group looked at using a metal alloy as the catalyst. To increase the surface area, they used nano-scale catalyst particles dispersed widely within the structure of mesoporous nanospheres, tiny sponge-like balls with thousands of channels running through them. The total surface area of these dispersed catalyst nanoparticles is roughly 100 times greater than the surface area you’d get with the same quantity of catalyst material in larger, macro-scale particles:
 energy :: sustainability :: ethanol :: biomass :: bioenergy :: biofuels :: gasification :: syngas :: catalyst :: synthetic biofuels ::
energy :: sustainability :: ethanol :: biomass :: bioenergy :: biofuels :: gasification :: syngas :: catalyst :: synthetic biofuels :: It is also important to control the chemical makeup of the syngas. Researchers at ISU's Center for Sustainable Environmental Technologies , or CSET, have spent several years developing fluidized bed gasifiers to provide reliable operation and high-quality syngas for applications ranging from replacing natural gas in grain ethanol plants to providing hydrogen for fuel cells.
Gasification to ethanol has received increasing attention as an attractive approach to reaching the Federal Renewable Fuel Standard of 36 billion gallons of biofuel. - Robert Brown, CSET director
The great thing about using syngas to produce ethanol is that it expands the kinds of materials that can be converted into fuels. You can use the waste product from the distilling process or any number of other sources of biomass, such as switchgrass or wood pulp. Basically any carbon-based material can be converted into syngas. And once we have syngas, we can turn that into ethanol, the researchers say.
The research is funded by the DOE’s Offices of Basic Energy Sciences and Energy Efficiency and Renewable Energy.
Ames Laboratory is a U.S. Department of Energy Office of Science laboratory operated for the DOE by Iowa State University. The Lab conducts research into various areas of national concern, including the synthesis and study of new materials, energy resources, high-speed computer design, and environmental cleanup and restoration .
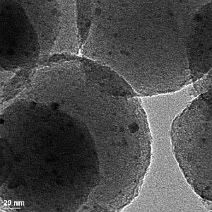
Image: In this transmission electron micrograph of the mesoporous nanospheres, the nano-scale catalyst particles show up as the dark spots. Using particles this small (~ 3nm) increases the overall surface area of the catalyst by roughly 100 times. Credit: Ames Lab.
Article continues
 --------------
--------------
 Mongabay, a leading resource for news and perspectives on environmental and conservation issues related to the tropics, has launched Tropical Conservation Science - a new, open access academic e-journal. It will cover a wide variety of scientific and social studies on tropical ecosystems, their biodiversity and the threats posed to them.
Mongabay, a leading resource for news and perspectives on environmental and conservation issues related to the tropics, has launched Tropical Conservation Science - a new, open access academic e-journal. It will cover a wide variety of scientific and social studies on tropical ecosystems, their biodiversity and the threats posed to them.









Thursday, August 14, 2008
Major research program for CO2-capture kicks off in Norway
SINTEF, the independent research organisation, The Norwegian University of Science and Technology and Aker Clean Carbon, the industrial technology company, on Thursday August 14 signed an agreement for an eight-year science and development programme called SOLVit. The programme has a total financial value of NOK 317 million.
Gassnova SF – the Norwegian government’s vehicle for CO2-management (capture, transport, injection and storage) – has approved financial support of NOK 34 million for the first phase of the project, which runs till the end of 2010.
Emissions from industry and power stations
The agreement concerns chemical processes that can capture CO2 from the process industry and emissions from coal and gas powered power stations. Within these sectors, it is estimated that the 4,000 largest facilities account for about 40 per cent of man-made CO2 emissions globally. The SOLVit programme aims to generate better and more cost effective processes and chemicals to manage CO2 emissions from these facilities.
International energy companies have been invited to participate in the programme. These will provide useful input from the perspective of the facility operator.
Test centre link-up
SOLVit makes SINTEF and NTNU able to consolidate the position as Europe’s leading science cluster for CO2-management. The programme includes building a large laboratory facility that will strengthen our standing in the international arena and improve our position in competition for financial support for scientific research from institutions such as the European Union, says Ms Unni Steinsmo, chief executive of SINTEF.
Results from the development research in the new laboratory in Trondheim will be tried out in test centres and in full-scale facilities already in the first phase of the programme. This makes SOLVit even more exciting.
Industrial competition
Aker Clean Carbon is heavily involved in competitions for CO2-capture projects in Norway and in the United Kingdom. Jan Roger Bjerkestrand, chief executive of Aker Clean Carbon, says the wide-ranging and thorough cooperation on scientific research under SOLVit to develop better and more energy effective chemicals for the capture and cleansing processes will strongly support the company’s standing in these competitions.
Aker Clean Carbon and SINTEF have together developed many chemical solutions based on amines, a chemical that has the ability to cleanse CO2. One of these solutions is already ready to use. Phase one of SOLVit will be used to test the other amine solutions under development by Aker Clean Carbon and SINTEF:
New laboratory in Trondheim
The programme also includes building a new laboratory at Tiller in Trondheim, which will cost NOK 42 million. SINTEF will provide NOK 25 million of the equity for the new laboratory, which will be situated next door to SINTEF’s multi-phase laboratory.
The lab will be a unique test centre for pilot projects, including a 30 metre tall tower and processing column that reached 25 metres high – identical to the height needed in full-scale industrial facilities. The lab will also be available for SINTEF’s domestic and international customers and partners.
Complete chain of laboratories
The SOLVit-programme will also involve the testing of chemicals and processes in a mobile capture facility, which has been developed by Aker Clean Carbon and is currently being built at Aker Verdal. The mobile facility is large enough to process parts of emissions from power stations and industrial sites in periods of several months at the time.
SINTEF and NTNU have already established laboratories for small-scale testing of CO2-capture. This means Norway will be among the few countries with a complete set of laboratories in this area, from testing in the lab to pilot runs at semi-industrial scale.
PhD and master students
Science and education go hand in hand in SOLVit. Using the programme as a basis, NTNU will offer positions to six doctoral candidates and ten master students within the subject of CO2-capture.
Joint financing
SOLVit has a budget of NOK 317 million and is led by Aker Clean Carbon. The financing is a joint effort by Aker, which is the main partner, and other industrial partners, Gassnova SF which participates through the public CLIMIT programme, and SINTEF and NTNU.
References:
Norwegian University of Science and Technology: Major research programme for CO2-capture - August 14, 2008.
Article continues
posted by Biopact team at 6:46 PM 0 comments links to this post
