A closer look at Direct Carbon Fuel Cells: the ultimate biomass conversion technology?
Last year, the director of the Department of Colloid Chemistry at the Max Planck Institute of Colloids and Interfaces, Prof Dr Markus Antonietti, developed an innovative technique with which any type of biomass can be converted into renewable and climate friendly 'designer coal'. Uses for the carbon are plenty, but professor Antonietti confessed that he and his researchers are part of a growing group of scientists who dream of a Direct Carbon Fuel Cell (DCFC) and a green carbon economy. As its name implies, a DCFC converts elemental carbon into electricity directly, and in a hyper-efficient way - the cells have almost twice the efficiency of most other types of fuel cells and double that of fossil fuel power plants.
What is more, the only byproduct of a DCFC's operation is very pure CO2 which can be contained in a concentrated stream and easily captured for downstream use or disposal. Because of the purity of the CO2 stream, capturing it would be far more cost-effective and efficient than capturing CO2 from conventional fossil fuel plants. Moreover, if the carbon feedstock for the fuel cell were to be derived from biomass, and the CO2 captured and sequestered, super-efficient carbon-negative electricity would be generated. That is: electricity the use of which results in the active removal of CO2 from the atmosphere (contrary to ordinary renewables like wind or solar, which merely prevent new emissions but don't go further than that). Quite a radical energy concept.
Now Prof Antonietti's dream is steadily becoming a reality, as a number of research institutions and companies are speeding up research and development into DCFCs. Let's have a closer look at these developments, which remain in their infancy.
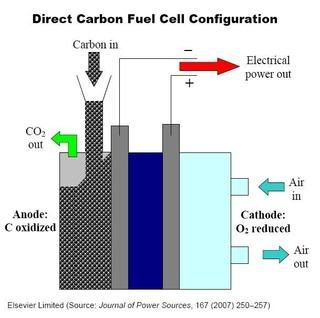
A fuel cell is an electrochemical device that efficiently converts a fuel's chemical energy directly to electrical energy without burning the fuel. However, instead of using gaseous fuels, as is typically done, DCFCs use aggregates of extremely fine (10- to 1,000-nanometer-diameter) carbon particles distributed in a mixture of molten lithium, sodium, Yttria-stabilized zirconia or potassium carbonate at a temperature of 600 to 850°C. The overall cell reaction is carbon and oxygen (from ambient air) forming carbon dioxide and electricity (schematic, click to enlarge).
The reaction yields 80 percent of the carbon–oxygen combustion energy as electricity, yet no burning of the carbon takes place. DCFCs for stationary applications provide up to 1 kilowatt of power per square meter of cell surface area — a rate sufficiently high for practical applications. Some developers are designing DCFCs for mobile applications that can deliver energy densities in the range of 1,000–2,000 Wh/kg, far higher than any advanced battery.
Benefits
DCFC technology has several potential benefits over other fuel cells. First, it can use a wide variety of very abundant low cost carbonaceous fuels including coal, coke, tar, biomass and organic waste. Conventional fuel cells typically operate on gaseous fuels. The fuel (natural gas, propane, ethanol, etc.) is reformed to a hydrogen syngas, which is fed into the fuel cell stack. The DCFC, however, can operate directly on solid carbon fuel, which is stable, easy to store, handle and transport. DCFCs don't require the construction of an entirely new and expensive infrastructure - which is the case for hydrogen - nor do they lose the energy needed to turn fuel into gas.
Secondly, unlike hydrogen or methanol fuel cells, DCFC use no catalyst or costly noble metals like platinum. This cuts costs, and should increase reliability. The design of several fuel cell stack types is relatively simple, with costs expected to be $250/m2 of cell area depending on manufacturing components. Together with the balance of the system, researchers and companies put the total projected cost at a target of around $1000/kW. Given the abundance and low cost of the fuel, operating DCFCs would be by far the least costly of all fuel cell systems. In a carbon constrained world, with incentives to capture and store CO2, and with a carbon price, capturing and storing CO2 from DCFCs would be far less costly than doing the same at conventional fossil fuel plants.
Thirdly, DCFC are much more efficient than any other type of fuel cell and power plant. At high temperatures (more than 600 °C), the carbon fuel is electro-oxidized to CO2 at the anode compartment creating electricity. The benefit of converting solid carbon directly to electricity enables the efficiency to be around 80 percent - experimentally verified -, well above that of other fuel cells, and double that of conventional steam power plants. Routinely, a DCFC converts 80% of the heat that would have been liberated by combustion into electric power instead. This increased efficiency results in a beneficial payoff for DCFC development, as well as a reduction of CO2 emissions to about one-tenth of that of a modern coal firing power plant. When biomass is used as the feedstock, CO2 emissions are close to zero, and if the greenhouse gas is captured and stored, the energy becomes carbon-negative.
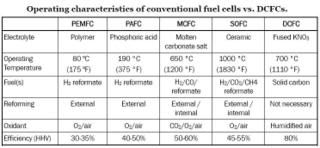
Table 1 outlines the operating characteristics of conventional fuel cells versus DCFCs (click to enlarge).
Biomass as an ideal feedstock
 DCFC's can use a large number of carbon-rich fuels, but organic waste and biomass are at the center of the attention because they are renewable and clean, but also because they can be turned into the purest carbon fuel. The overall process of producing electricity in a DCFC from biomass gains efficiency by its simplicity. It involves only two steps: (1) drying (and/or pyrolysis, or hydrothermal carbonization) to obtain char, and (2) feeding the resulting fuel directly to the DCFC. Drying and/or pyrolysis or conversion into char via hydrothermal carbonization is required to create a carbon-rich particulate solid that can be fed to the DCFC fuel cell to produce power:
DCFC's can use a large number of carbon-rich fuels, but organic waste and biomass are at the center of the attention because they are renewable and clean, but also because they can be turned into the purest carbon fuel. The overall process of producing electricity in a DCFC from biomass gains efficiency by its simplicity. It involves only two steps: (1) drying (and/or pyrolysis, or hydrothermal carbonization) to obtain char, and (2) feeding the resulting fuel directly to the DCFC. Drying and/or pyrolysis or conversion into char via hydrothermal carbonization is required to create a carbon-rich particulate solid that can be fed to the DCFC fuel cell to produce power:
 energy :: sustainability :: biomass :: bioenergy :: biofuels :: charcoal :: pyrolysis :: hydrothermal carbonisation :: carbon :: direct carbon fuel cell :: carbon capture :: carbon-negative :: efficiency :: climate change ::
energy :: sustainability :: biomass :: bioenergy :: biofuels :: charcoal :: pyrolysis :: hydrothermal carbonisation :: carbon :: direct carbon fuel cell :: carbon capture :: carbon-negative :: efficiency :: climate change ::
The choice between drying or pyrolyzing the biomass before feeding it to the DCFC will depend on whether the energy contained in the waste gases resulting from the conversion of the dried biomass within the DCFC can be recovered efficiently, and whether the DCFC can be designed in a manner so that it is not fouled by the light gases and tars generated.
As a fuel, char produced from biomass and waste materials offers many benefits. It is inexpensive to produce and easy to store. Char is readily available to consumers worldwide from compacted beds with high-energy density particles. When combusted correctly, charcoal does not burden the atmosphere with CO2 emissions, and does not contribute to climate change. In contrast with fossil fuels, charcoal has no mercury, almost no sulfur, low nitrogen, and produces very little ash. It has high electrical conductivity, a large surface area, and many bonds that enable it to be very reactive at relatively modest temperatures.
DCFC developers favor fuels that are essentially pure carbon particles, with little inherent moisture, ash, sulfur, and nitrogen. Biomass from energy crops, waste paper products, structural wood, and a fraction of Municipal Solid Waste (MSW) can be converted into the type of fuel most highly valued by DCFC vendors by drying and pyrolysing.
DCFC Types
Several approaches to the development of DCFCs are underway. These can be grouped into three broad categories, depending on the type of electrolyte used.
DCFCs with a Molten Carbonate Electrolyte
Molten carbonate electrolytes are very good for DCFCs because they are highly conductive, have good stability when CO2 is present, and have an appropriate melting temperature for this application. The cell voltage is formed at the anode side and consumed at the cathode side, and there is an influence on the cell voltage by this partial pressure. Simulations have given results showing the system to be able to reach a net electrical efficiency of up to 78 percent.
DCFCs with a Molten Hydroxide Electrolyte
Molten hydroxides are very beneficial as electrolytes. They have a higher ionic conductivity and a higher activity of the carbon electrochemical oxidation. This results in a lower overpotential and a higher carbon oxidation rate, as well as a much lower operation temperature of about 600 °C. This decreases the cost as it allows the use of less expensive materials.
During carbon electro-oxidation in this type of fuel cell, there is the formation of carbonates. They undergo both a chemical process and an electro-chemical process. This fuel cell uses a pure graphite cylindrical rod, which acts as the anode and the fuel. It is immersed into molten sodium hydroxide and is served at the same time as the cathode. The cell is fed humidified air through a gas distributor in the bottom of the container.
To optimize the performance of the cell, one must look at the cathode material, air flow rate, operating temperature, and fuel cell scale. They system can be further optimized by changing the cell design, the electrode material, and the operating conditions.
DCFCs with YSZ-based Solid Electrolyte
The Yttria-Stabilized Zirconia (YSZ) design combines advances in the solid oxide and molten carbonate fuel cell technologies. Their components include a U-tube consisting of a metal mesh cathode current collector, a cathode layer, an electrolyte later, and a metal mesh anode current collector. This structure is immersed into a liquid anode made of a mixture of molten elements and carbon particles. When this mixture is stirred causing a flow mode, the fuel cell operates better since there is an increase contact between the carbon particles and the anode current collector, which enhances mass transport.
Current research
Around seven teams in the U.S. are actively investing in DCFC research and development. European and Japanese researchers are doing so as well, but information is limited.
Amongst the U.S. teams can be found researchers from Akron University, CellTech Power, Contained Energy, Direct Carbon Technologies, Scientific Applications & Research Associates (SARA), SRI, and the University of Hawaii.
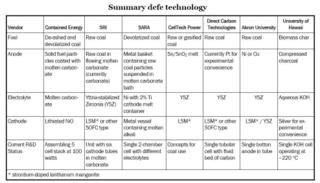
The following table summarises their approaches to DCFC development (click to enlarge).
The Lawrence Livermore National Laboratory (LLNL) has a development program for the DCFC and recently made a breakthrough. The technology was the result of a two-year study funded by the Laboratory Directed Research and Development Program, and led to a DCFC that pushes the efficiency of using fossil fuels for generating electricity far closer to theoretical limits than ever before. Rights to the patented LLNL process have been acquired by Contained Energy.
The following are schematic illustrations and explanations of different DCFC types currently under development.
Contained Energy has exclusively licensed the DCFC technology developed by John Cooper at Lawrence Livermore National Laboratory (LLNL). The cathode in this technology is essentially a molten carbonate cathode, while the anode is a slurry of disordered carbon fuel and a carbonate eutectic. Under a Cooperative Research and Development Agreement (CRADA), Contained Energy engaged LLNL to develop the initial prototypes of its generation design; a single cell of 15W–30W output, and a five-cell bipolar stack of 75W–150W output.
This design has an area-specific resistance (ASR) of 0.69 Ω/cm2, which corresponds to a maximum theoretical power density for the cell of 280 mW/cm2. However, with variances in individual cell performance in the stack, and with realistic losses from interconnects, Contained Energy is targeting a maximum gross power density of 140-200 mW/cm2. Such a cell has operated for a period of 7 days.
In early development work at LLNL, the cathode was identified as the rate limiting subsystem. Under the work during the CRADA, the cathode has been improved with new materials and a proprietary activation procedure. Having improved the cathode, the separator is now the limiting constraint in the system, apparently due to a change in the chemical composition of the fabric YSZ separator produced by the supplier. The supplier is working to correct the problem. Meanwhile Contained Energy is also developing alternative separators that should have the same or superior performance characteristics.
Contained Energy is transferring the results of this CRADA to their devel-opment facility in Cleveland, OH. Contained Energy is simultaneously developing a different design for mobile applications that can deliver energy density in the range of 1,000–2,000 Wh/kg.
All of Akron University's work described to date has been carried out on button cells lo-cated in a tubular apparatus. Most of the effort has been to test various combinations of anode and cathode catalysts. Typical experiments consist of placing a small amount of either raw coal or devolatilized coal on the button cell and either heating it up or dropping coal directly into a pre-heated cell. Test temperatures are normally in the range of 750–850 °C.
Power densities in the range of 50–150 mW/cm2 have been obtained during the relatively short test duration of a few hours. Ash build-up on the surface of the button cell reduces power density, but removing the loose ash from the cell surface and allowing fresh carbon to reach the surface restores power density to previous levels.
The first experiments with a fluidized bed of solid carbon fuel (i.e., synthetic carbon, coal and almond shell) particles provided peak power out-puts of 1-2 mW/cm2 at 900 °C with a flowing CO2 or He atmosphere. These experiments were done with an initial charge of 30 grams of solid carbon fuel and ran for more than 20 hrs. In some cases, erosion has been observed with delamination of the platinum anode.
Benchmarking experiments done for comparison reasons with gaseous fuels (3% H2 and 100 percent CO) in the absence of solid fuel in the bed and using the same cells similarly gave peak power densities of 1-2 mW/cm2. In both solid and gaseous fuel cases, the fuel cell behavior was dominated by ohmic loses due mostly to the high resistance of the thick partially sta-bilized zirconia (PSZ) tubular electrolyte employed in these experiments.
In contrast, experiments at those same conditions in the tubular reactor, with the synthetic carbon placed on button cells (featuring thin yttria sta-bilized zirconia ([YSZ] electrolyte wafers with Ni/YSZ cermet anodes) provided by Ceramatec (Salt Lake City, UT) and agitated by a flowing CO2 stream produced a peak power density in excess of 140 mW/cm2, which deteriorated in time due to sulfur interaction with the Ni anode. Similar experiments using fluidized coal in flowing He gas with other button cells gave peak power densities in excess of 40 mW/cm2, which also decayed in time. Again, benchmarking tests on these same button cells using gaseous fuels only gave comparable power densities. These results pointed to the importance of the microstructure, stability, and catalycity of the anode and its impact on cell performance.
In all cases, gas analyses of the reaction products verified oxygen balance around the cell, and indicated that all oxygen, supplied electro-chemically through the solid electrolyte into the solid fuel bed, is ac-counted for in the form of CO and CO2 in the flue stream. These prelimi-nary results demonstrated for the first time that one can electrochemically convert solid carbonaceous fuels into electricity in a single step inside a fluidized bed reactor.
CellTech Power is developing a technology that uses a liquid tin anode in a solid oxide fuel cell. This system oxidizes molten tin (Sn) to tin oxides (such as SnO2) in the anode layer by oxygen ions produced in the cathode. The ions transit a typical Yttria-stabilized Zirconia (YSZ) electro-lyte to reach the anode such that electrons are released. Electricity can be produced directly by oxidizing Sn like a battery.
The SnO2 can be reduced back to Sn by carbon-containing solids or any reducing gases consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur that enters the anode. During the Sn regeneration, the device operates like a fuel cell. The Sn anode is not poisoned by sulfur. With a cell open circuit voltage (OCV) of 0.8V, the CO/CO2 ratio is 0.2 in the anode effluent gas. Maintaining cell voltage (OCV) above 0.8V keeps the dissolved SnO2 concentration in the molten Sn at a level where precipitation of the oxide does not occur. This means that the CO-containing gaseous effluent that leaves the cell must be oxi-dized to complete the conversion of CO to CO2.
Several years ago, with $15 million raised from venture capital and private sources, CellTech built two 1 kW Gen 2 units fueled by natural gas, which operated for more than 2000 hrs continuously. In those Gen 2 units, the natural gas was conditioned to a stream also containing CO and hydrogen and fed to the Sn anode. During 2005-2006, with Defense Advanced Re-search Projects Agency (DARPA) funding, CellTech developed Gen 3.0 cells and stacks allowing direct conversion of waste packaging materials and JP-8 into electricity. Before 2005, the key limitations of this system had been low power density (with levels of 40 mW/cm2 with hydrogen fuel and 20 mW/cm2 with carbon/JP-8 fuel) and difficulty in manufacturing. These power densities had been deemed too low for portable and mobile power generation. With support from DARPA/Army recently in place, CellTech is developing a Gen 3.1 (2007) cell architecture for direct JP-8 conversion with improved power density. They have modified the porous media to allow higher mass transfer rates of heavy fuel molecules flowing to the anode and are developing a high electrical conductance tubular cathode.
In 2006, CellTech demonstrated power densities of 160 mW/cm2 for hydrogen and 80 mW/cm2 for JP-8. The Gen 3.1 design is expected to provide approximately four times reduction in weight and volume over the previous Gen 3.0. Gen 3.1 is projected to become competitive for number of portable and mobile applications such as military field battery chargers. The mid-term power density target for direct JP-8 conversion is 200 mW/cm2 (2008-2010); at this level the direct JP-8 conversion liquid Sn system becomes a formidable competitor for kilowatt or sub-kilowatt applications.
CellTech Power has several concepts of how to generate power from coal with this system, but has not completed a detailed flowsheet analysis. One approach involves feeding coal to a molten Sn bath anode to reduce SnO2 to Sn, then transferring the molten Sn to the cell arrays for oxidation to SnO2 and power production. Another concept is to use a fluidized bed of coal to take advantage of volatiles in coal, in which carbon in the coal is reacted with hot recycled CO2 and water to produce a CO-rich gas, which is then fed to the cell array to produce power.
Charcoal has been used as the feedstock for a low temperature aqueous carbonate fuel cell that has operated as high as 245 °C. At this temperature the cell offered an open circuit voltage of 0.57 V and a short circuit current of 43.6 mA/cm2. At 220 °C, the power density was 6.3 mW/cm2. One possible explanation for the relatively low open circuit voltages resulted from the formation of carbon oxides on the anode that were accompanied by the release of CO2.
Thermodynamically, oxygen reduction at the cathode is more favorable at temperatures below 200 °C, however, improved anode performance could result from a higher temperature that could combust the carbon oxides ac-cumulated on the bicarbon anode material. Therefore, performance could be markedly improved if a split cell could be developed in which the cathode could be operated at below 200 °C and the anode at above 240 °C.
SARA has evolved a new concept that uses different salts in two chambers separated by a porous separator plate. The cathode chamber contains molten potassium (KOH) or sodium hy-droxide (NaOH). Better results have been obtained with KOH. Moist air is bubbled into this chamber where the oxygen picks up electrons, resulting in the formation of OH- ions, which then transport through the separator membrane to enter the anode chamber. A basket of solid fuel particles is suspended in molten metal carbonates in the anode chamber. The OH- ions react with the solid fuel to produce CO3-2 ions and electrons. The CO3-2 ions also react with the coal to produce CO2 and electrons.
SARA recently observed that the electrolyte was stable over the course of a 500-hr experiment. A stackable design concept has been developed. They stated that the major challenges are the separator material and design, corrosion, and operating temperature. Power density numbers for this system are difficult to compare to the other systems reviewed because those numbers are based on the area of the separator rather an anode or cathode area.
Finally, one company, SRI has developed a concept based on feeding coal (as well as other carbon sources such as tar, biomass and waste paper/plastic) as a carbon rich solid fuel to a flowing molten salt, such as alkali metal carbonates. That mixture forms an electrically conducting anode when the carbon concentration reaches a value between 30 and 40 percent. Air is fed to a conventional SOFC cathode (typically strontium-doped lanthanum manganite [LSM]) which provides the oxygen ions that migrate through a solid oxide electrolyte (typically YSZ) and react with the solid fuel to produce electricity and CO2. SRI currently is operating a batch system which has up to six cathode/electrolyte tubes inserted into a single molten salt bath. Three types of tubes are being used: simplified, sub-scale, and full-scale
For the experiments conducted to date, the solid fuel is mixed with salt powder and the dry mixture is then dropped into the apparatus, which is then heated to its operating temperature of 800-950 °C. Power densities of approximately 300 mW/cm2 have been achieved. Lifetimes in excess of 1200 hours have also been demonstrated. A design for a 40 kW power system has been completed.
Integrating biomass pyrolysis and electricity production
As outlined above, there are several techniques for the production of a solid, carbon-rich, particulate fuel from various carbon-rich biomass and organic waste resources. These processes themselves generate heat and gases that can be utilized for the production of power. The question now is whether to integrate this operation with the DCFC's operation into a continuously operating system or to disperse the fuel preparation and electricity production functions to separate locations.
In the integrated case, it is necessary to transport the fuel to a central location and then transmit and distribute electricity from that location to a variety of users. The integrated approach offers an opportunity for maximum energy efficiency as a result of integration between the fuel preparation and fuel consumption operations as well as the opportunity to use that waste energy (thermal and methane) in other buildings or processing plants that could be co-located with the integrated unit.
Figure 2 (click to enlarge) shows a system with the potential for the highest energy efficiency and best opportunity for energy integration with co-located facilities. In this concept, feed material is dried at about 149 °C to drive water off from the wet biomass/MSW feed material. The dried feed is then pyrolized at 371 °C to drive off methane and carbon dioxide and produce char which is fed to the DCFC. Hot, CO2-rich anode product gas is recycled from the DCFC to the fuel dryer and pyrolyzer to provide the heat energy needed for those operations.
Excess energy in the pyrolyzer waste gas and in the CO2 rich anode-off gas can be used for steam generation and perhaps used in co-located energy consuming facilities.
Overall process efficiency
In a recent overview of DCFC technologies, researchers at the ERDC-CERL Fuel Cell Program of the U.S. Army Corps of Engineers undertook a basic calculation of the overall efficiency of the process to go from raw carbon-rich, biomass to electricity. Since the work of all the teams involved in developing DCFC technology is at very early stages of development, it is not likely that a fully integrated stack and fuel cell power plant will achieve the maximum theoretical efficiency of 80 percent that occurs in a single cell.
The researchers therefor assumed that the efficiency of converting the chemical energy (Higher Heating Value or HHV basis) in the dry carbon in the fuel to AC electricity in the DCFC is 65 percent. (Typical pulverized coal power plants convert the chemical energy in coal (on the same HHV basis) to electricity at 30–35 percent efficiency.)
Wood received in a power plant is assumed to contain 45 percent moisture. The typical carbon composition of dry-wood is assumed to be 50 percent. The heat of combustion of carbon when CO2 is the only product is 14,087 Btu/pound. Therefore the amount of pure carbon required to produce 1 MWH of electricity is 372 lb. If dry wood contains 50 percent carbon, then 372 lb of carbon is contained in 744 lb of dry wood.
Assuming that wet wood contains 45 percent moisture, then the amount of wet wood required for 168.7kg (372 lb) of carbon or 337.5kg (744 lb) of dry wood is 613.7kg (1353 lb). It follows that the amount of water contained in the wet wood is 276.2kg (609 lb). Drying wood by evaporation requires approximately 1000 Btu of heat per pound of water evaporated. Removing 276.2kg (609 lb) of water from wet wood requires 642.5MJ (609,000 Btu) of energy.
Converting 168.7kg (372 lb) of carbon into 1 MWH of electricity in a DCFC liberates 1,939 MJ (1,838,000 Btu) of waste heat in the fuel cell. The amount of energy contained in 1 MWH of electricity is 3,599,851Kj, from which it follows that the waste heat available is 1,939,192.9 kJ/MWH.
To maintain the fuel cell at its constant operating temperature of approxi-mately 760 °C (1400 °F), this waste heat must be removed from the DCFC system both by heating up the reactants that are fed to it, and cooling and recycling the gas product from the cell.
The waste heat available of 1,939 MJ/MWH (1,838,000 Btu/MWH) is far more than the 642 MJ/MWH (609,000Btu/MWH) required for wet wood drying. It can therefore be used to supply the heat needed for evaporation of the water from the wet wood.
The pyrolysis step also liberates a significant amount of methane. A reasonable assumption is that 10 percent of the mass of dry wood fed to a pyrolysis reactor will be produced as methane. Therefore, 613.7kg (1353 lb) of wet wood containing 744 lb of dry wood will yield about 33.7kg (74.4 lb) of methane. This amount of methane contains about 2,110 MJ (2,000,000 Btu’s) of energy, which could be used for drying or pyrolysis, or which could be exported for external uses.
Capturing CO2: green extreme
Countries with extensive coal reserves will continue to use coal as their primary source of electricity for many years to come. Biomass will be co-fired or used in dedicated power plants more often, but coal is set to remain the most widely used fuel. However, today's coal-fired power plants convert coal into electricity with relatively low efficiency, often not higher than 30 to 35 percent. In addition, coal is a source of toxic emissions, greenhouse gases and heavy metal pollutants when used in traditional combustion power plants. For coal dependent countries to enter a more environmentally sustainable and economically feasible way, a clean, efficient and direct process to convert coal into electrical energy is needed. This is where the DCFC can play a major role.
The CO2 from conventional fossil fuel power plants can be captured via a range of processes - pre-combustion, oxyfuel or post-combustion capture - but separating the gas from other flue or process gases is complex, energy demanding and requires expensive membranes or adsorption technologies. Nonetheless, large funds are being poured into such carbon capture and storage (CCS) research projects.
DCFCs have the major advantage that the CO2 stream they generate is extremely pure, allowing the climate destructive gas to be captured with ease. Legislation allowing CCS and a carbon price would give DCFCs a competitive advantage because they significantly lower the CO2 capture cost. If carbon-dioxide-capturing costs are included, DCFC system costs are estimated to be up to 50 percent less than those of current fossil fuel plants.
What is more, the fact that DCFCs can operate on the carbon contained in biomass, allows for the development of the most radically green type of energy system imaginable: one that results in negative emissions.
This is possible because as biomass grows it sequesters atmospheric CO2. When this gas is captured at the DCFC and then stored in geological formations such as depleted oil and gas fields or saline aquifers, the biomass actively removed CO2 from the atmosphere. Instead of being merely carbon-neutral (as other types of renewable energy are), the energy thus obtained would be carbon-negative.
 Biopact readers are aware of research which shows that when biomass is burned in integrated gasification combined cycle (IGCC) plants, with the CO2 captured and stored, that negative emissions as high as minus 1000 grams of CO2 per KWh can be obtained. Solar photovoltaic (+100grams), wind (+30grams), non-CCS biomass (+30grams) and nuclear (+10-20 grams) are all carbon positive (graph, click to enlarge). Biomass burned in an IGCC coupled to CCS goes way beyond that and removes historic CO2 from the atmosphere. It is thus by far the most radical tool in the climate fight.
Biopact readers are aware of research which shows that when biomass is burned in integrated gasification combined cycle (IGCC) plants, with the CO2 captured and stored, that negative emissions as high as minus 1000 grams of CO2 per KWh can be obtained. Solar photovoltaic (+100grams), wind (+30grams), non-CCS biomass (+30grams) and nuclear (+10-20 grams) are all carbon positive (graph, click to enlarge). Biomass burned in an IGCC coupled to CCS goes way beyond that and removes historic CO2 from the atmosphere. It is thus by far the most radical tool in the climate fight.
However, IGCCs are only moderately efficient, because they rely on the combustion of fuels; a considerable amount of the energy generated would be needed to capture the CO2, thus lowering the overall systems efficiency of IGCCs + CCS. DCFCs in contrast are up to twice as efficient and would thus allow the capture of CO2 in a far more efficient manner still. There are no calculations showing the overall efficiency of capturing CO2 from biomass by using part of the electricity generated in a DCFC, but it can be safely assumed that the process would be far more efficient than the same operation performed in a IGCC.
In short, the confluence of different factors in operating direct carbon fuel cells - the purity of the CO2 stream and the efficiency of the electricity generating process - makes it possible to imagine what is possibly the cleanest and greenest form of electricity production imaginable: efficiently generated negative emissions energy. Efficient DCFCs could help clean up the atmosphere and help us prevent climate change in the most drastic way.
References:
A large number of older, but key articles can be found online at the National Technology Energy Library: Direct Carbon Fuel Cell Workshop.
University of Hawaii; Hawaii Natural Energy Institute - Renewable Resources Research Laboratory: Biocarbon Fuel Cells.
Ronald H. Wolk, Scott Lux, Stacy Gelber, and Franklin H. Holcomb, Direct Carbon Fuel Cells: Converting Waste to Electricity [*.pdf], U.S. Army Corps of Engineers: ERDC-CERL Fuel Cell Program, September 2007
Antal, Michael J, Performance of a First Generation Aqueous Alkaline Biocarbon Fuel Cell, Ind. Eng. Chem. Res., 46 (3), 734 -744, 2007. DOI: 10.1021/ie061202s S0888-5885(06)01202-4
Cao, D., Y. Sun, G. Wang, Direct Carbon Fuel Cell: Fundamentals and Recent Developments, Journal of Power Sources, vol 167, No. 2, pp 250-257, May 15, 2007.
Hackett, Gregory A., John W. Zondlo, Robert Svensson. “Evaluation of Carbon Materials for Use in a Direct Carbon Fuel Cell,” Journal of Power Sources, vol 168, No. 1, pp 111-118, May 25, 2007.
Heydorn, Barbara, and Steven Crouch-Baker, “Direct Carbon Conversion: Progressions of Power,” The Fuel Cell Review, 2006.
Lawrence Livermore National Laboratory: Direct Carbon Fuel Cells.
SRI International: SRI International Presents Novel Direct Carbon Fuel Cell Technology at Industry Event - November, 11, 2005
SARA: Direct Carbon Fuel Cell.
Biopact: Back to black: hydrothermal carbonisation of biomass to clean up CO2 emissions from the past - May 26, 2007
What is more, the only byproduct of a DCFC's operation is very pure CO2 which can be contained in a concentrated stream and easily captured for downstream use or disposal. Because of the purity of the CO2 stream, capturing it would be far more cost-effective and efficient than capturing CO2 from conventional fossil fuel plants. Moreover, if the carbon feedstock for the fuel cell were to be derived from biomass, and the CO2 captured and sequestered, super-efficient carbon-negative electricity would be generated. That is: electricity the use of which results in the active removal of CO2 from the atmosphere (contrary to ordinary renewables like wind or solar, which merely prevent new emissions but don't go further than that). Quite a radical energy concept.
Now Prof Antonietti's dream is steadily becoming a reality, as a number of research institutions and companies are speeding up research and development into DCFCs. Let's have a closer look at these developments, which remain in their infancy.
A fuel cell is an electrochemical device that efficiently converts a fuel's chemical energy directly to electrical energy without burning the fuel. However, instead of using gaseous fuels, as is typically done, DCFCs use aggregates of extremely fine (10- to 1,000-nanometer-diameter) carbon particles distributed in a mixture of molten lithium, sodium, Yttria-stabilized zirconia or potassium carbonate at a temperature of 600 to 850°C. The overall cell reaction is carbon and oxygen (from ambient air) forming carbon dioxide and electricity (schematic, click to enlarge).
The reaction yields 80 percent of the carbon–oxygen combustion energy as electricity, yet no burning of the carbon takes place. DCFCs for stationary applications provide up to 1 kilowatt of power per square meter of cell surface area — a rate sufficiently high for practical applications. Some developers are designing DCFCs for mobile applications that can deliver energy densities in the range of 1,000–2,000 Wh/kg, far higher than any advanced battery.
Benefits
DCFC technology has several potential benefits over other fuel cells. First, it can use a wide variety of very abundant low cost carbonaceous fuels including coal, coke, tar, biomass and organic waste. Conventional fuel cells typically operate on gaseous fuels. The fuel (natural gas, propane, ethanol, etc.) is reformed to a hydrogen syngas, which is fed into the fuel cell stack. The DCFC, however, can operate directly on solid carbon fuel, which is stable, easy to store, handle and transport. DCFCs don't require the construction of an entirely new and expensive infrastructure - which is the case for hydrogen - nor do they lose the energy needed to turn fuel into gas.
Secondly, unlike hydrogen or methanol fuel cells, DCFC use no catalyst or costly noble metals like platinum. This cuts costs, and should increase reliability. The design of several fuel cell stack types is relatively simple, with costs expected to be $250/m2 of cell area depending on manufacturing components. Together with the balance of the system, researchers and companies put the total projected cost at a target of around $1000/kW. Given the abundance and low cost of the fuel, operating DCFCs would be by far the least costly of all fuel cell systems. In a carbon constrained world, with incentives to capture and store CO2, and with a carbon price, capturing and storing CO2 from DCFCs would be far less costly than doing the same at conventional fossil fuel plants.
Thirdly, DCFC are much more efficient than any other type of fuel cell and power plant. At high temperatures (more than 600 °C), the carbon fuel is electro-oxidized to CO2 at the anode compartment creating electricity. The benefit of converting solid carbon directly to electricity enables the efficiency to be around 80 percent - experimentally verified -, well above that of other fuel cells, and double that of conventional steam power plants. Routinely, a DCFC converts 80% of the heat that would have been liberated by combustion into electric power instead. This increased efficiency results in a beneficial payoff for DCFC development, as well as a reduction of CO2 emissions to about one-tenth of that of a modern coal firing power plant. When biomass is used as the feedstock, CO2 emissions are close to zero, and if the greenhouse gas is captured and stored, the energy becomes carbon-negative.
Table 1 outlines the operating characteristics of conventional fuel cells versus DCFCs (click to enlarge).
Biomass as an ideal feedstock
 DCFC's can use a large number of carbon-rich fuels, but organic waste and biomass are at the center of the attention because they are renewable and clean, but also because they can be turned into the purest carbon fuel. The overall process of producing electricity in a DCFC from biomass gains efficiency by its simplicity. It involves only two steps: (1) drying (and/or pyrolysis, or hydrothermal carbonization) to obtain char, and (2) feeding the resulting fuel directly to the DCFC. Drying and/or pyrolysis or conversion into char via hydrothermal carbonization is required to create a carbon-rich particulate solid that can be fed to the DCFC fuel cell to produce power:
DCFC's can use a large number of carbon-rich fuels, but organic waste and biomass are at the center of the attention because they are renewable and clean, but also because they can be turned into the purest carbon fuel. The overall process of producing electricity in a DCFC from biomass gains efficiency by its simplicity. It involves only two steps: (1) drying (and/or pyrolysis, or hydrothermal carbonization) to obtain char, and (2) feeding the resulting fuel directly to the DCFC. Drying and/or pyrolysis or conversion into char via hydrothermal carbonization is required to create a carbon-rich particulate solid that can be fed to the DCFC fuel cell to produce power: energy :: sustainability :: biomass :: bioenergy :: biofuels :: charcoal :: pyrolysis :: hydrothermal carbonisation :: carbon :: direct carbon fuel cell :: carbon capture :: carbon-negative :: efficiency :: climate change ::
energy :: sustainability :: biomass :: bioenergy :: biofuels :: charcoal :: pyrolysis :: hydrothermal carbonisation :: carbon :: direct carbon fuel cell :: carbon capture :: carbon-negative :: efficiency :: climate change :: The choice between drying or pyrolyzing the biomass before feeding it to the DCFC will depend on whether the energy contained in the waste gases resulting from the conversion of the dried biomass within the DCFC can be recovered efficiently, and whether the DCFC can be designed in a manner so that it is not fouled by the light gases and tars generated.
As a fuel, char produced from biomass and waste materials offers many benefits. It is inexpensive to produce and easy to store. Char is readily available to consumers worldwide from compacted beds with high-energy density particles. When combusted correctly, charcoal does not burden the atmosphere with CO2 emissions, and does not contribute to climate change. In contrast with fossil fuels, charcoal has no mercury, almost no sulfur, low nitrogen, and produces very little ash. It has high electrical conductivity, a large surface area, and many bonds that enable it to be very reactive at relatively modest temperatures.
DCFC developers favor fuels that are essentially pure carbon particles, with little inherent moisture, ash, sulfur, and nitrogen. Biomass from energy crops, waste paper products, structural wood, and a fraction of Municipal Solid Waste (MSW) can be converted into the type of fuel most highly valued by DCFC vendors by drying and pyrolysing.
DCFC Types
Several approaches to the development of DCFCs are underway. These can be grouped into three broad categories, depending on the type of electrolyte used.
DCFCs with a Molten Carbonate Electrolyte
Molten carbonate electrolytes are very good for DCFCs because they are highly conductive, have good stability when CO2 is present, and have an appropriate melting temperature for this application. The cell voltage is formed at the anode side and consumed at the cathode side, and there is an influence on the cell voltage by this partial pressure. Simulations have given results showing the system to be able to reach a net electrical efficiency of up to 78 percent.
DCFCs with a Molten Hydroxide Electrolyte
Molten hydroxides are very beneficial as electrolytes. They have a higher ionic conductivity and a higher activity of the carbon electrochemical oxidation. This results in a lower overpotential and a higher carbon oxidation rate, as well as a much lower operation temperature of about 600 °C. This decreases the cost as it allows the use of less expensive materials.
During carbon electro-oxidation in this type of fuel cell, there is the formation of carbonates. They undergo both a chemical process and an electro-chemical process. This fuel cell uses a pure graphite cylindrical rod, which acts as the anode and the fuel. It is immersed into molten sodium hydroxide and is served at the same time as the cathode. The cell is fed humidified air through a gas distributor in the bottom of the container.
To optimize the performance of the cell, one must look at the cathode material, air flow rate, operating temperature, and fuel cell scale. They system can be further optimized by changing the cell design, the electrode material, and the operating conditions.
DCFCs with YSZ-based Solid Electrolyte
The Yttria-Stabilized Zirconia (YSZ) design combines advances in the solid oxide and molten carbonate fuel cell technologies. Their components include a U-tube consisting of a metal mesh cathode current collector, a cathode layer, an electrolyte later, and a metal mesh anode current collector. This structure is immersed into a liquid anode made of a mixture of molten elements and carbon particles. When this mixture is stirred causing a flow mode, the fuel cell operates better since there is an increase contact between the carbon particles and the anode current collector, which enhances mass transport.
Current research
Around seven teams in the U.S. are actively investing in DCFC research and development. European and Japanese researchers are doing so as well, but information is limited.
Amongst the U.S. teams can be found researchers from Akron University, CellTech Power, Contained Energy, Direct Carbon Technologies, Scientific Applications & Research Associates (SARA), SRI, and the University of Hawaii.
The following table summarises their approaches to DCFC development (click to enlarge).
The Lawrence Livermore National Laboratory (LLNL) has a development program for the DCFC and recently made a breakthrough. The technology was the result of a two-year study funded by the Laboratory Directed Research and Development Program, and led to a DCFC that pushes the efficiency of using fossil fuels for generating electricity far closer to theoretical limits than ever before. Rights to the patented LLNL process have been acquired by Contained Energy.
The following are schematic illustrations and explanations of different DCFC types currently under development.
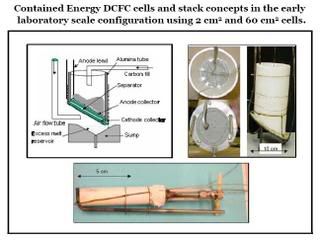
Early laboratory configuration of Contained Energy's fuel cell, based on LLNL's design
Contained Energy has exclusively licensed the DCFC technology developed by John Cooper at Lawrence Livermore National Laboratory (LLNL). The cathode in this technology is essentially a molten carbonate cathode, while the anode is a slurry of disordered carbon fuel and a carbonate eutectic. Under a Cooperative Research and Development Agreement (CRADA), Contained Energy engaged LLNL to develop the initial prototypes of its generation design; a single cell of 15W–30W output, and a five-cell bipolar stack of 75W–150W output.
This design has an area-specific resistance (ASR) of 0.69 Ω/cm2, which corresponds to a maximum theoretical power density for the cell of 280 mW/cm2. However, with variances in individual cell performance in the stack, and with realistic losses from interconnects, Contained Energy is targeting a maximum gross power density of 140-200 mW/cm2. Such a cell has operated for a period of 7 days.
In early development work at LLNL, the cathode was identified as the rate limiting subsystem. Under the work during the CRADA, the cathode has been improved with new materials and a proprietary activation procedure. Having improved the cathode, the separator is now the limiting constraint in the system, apparently due to a change in the chemical composition of the fabric YSZ separator produced by the supplier. The supplier is working to correct the problem. Meanwhile Contained Energy is also developing alternative separators that should have the same or superior performance characteristics.
Contained Energy is transferring the results of this CRADA to their devel-opment facility in Cleveland, OH. Contained Energy is simultaneously developing a different design for mobile applications that can deliver energy density in the range of 1,000–2,000 Wh/kg.
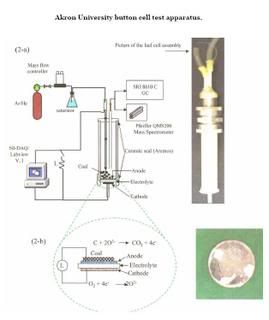
Akron University's fuel cell
All of Akron University's work described to date has been carried out on button cells lo-cated in a tubular apparatus. Most of the effort has been to test various combinations of anode and cathode catalysts. Typical experiments consist of placing a small amount of either raw coal or devolatilized coal on the button cell and either heating it up or dropping coal directly into a pre-heated cell. Test temperatures are normally in the range of 750–850 °C.
Power densities in the range of 50–150 mW/cm2 have been obtained during the relatively short test duration of a few hours. Ash build-up on the surface of the button cell reduces power density, but removing the loose ash from the cell surface and allowing fresh carbon to reach the surface restores power density to previous levels.
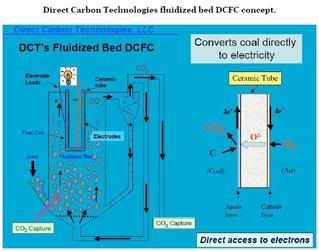
Direct Carbon Technologies DCFC
The first experiments with a fluidized bed of solid carbon fuel (i.e., synthetic carbon, coal and almond shell) particles provided peak power out-puts of 1-2 mW/cm2 at 900 °C with a flowing CO2 or He atmosphere. These experiments were done with an initial charge of 30 grams of solid carbon fuel and ran for more than 20 hrs. In some cases, erosion has been observed with delamination of the platinum anode.
Benchmarking experiments done for comparison reasons with gaseous fuels (3% H2 and 100 percent CO) in the absence of solid fuel in the bed and using the same cells similarly gave peak power densities of 1-2 mW/cm2. In both solid and gaseous fuel cases, the fuel cell behavior was dominated by ohmic loses due mostly to the high resistance of the thick partially sta-bilized zirconia (PSZ) tubular electrolyte employed in these experiments.
In contrast, experiments at those same conditions in the tubular reactor, with the synthetic carbon placed on button cells (featuring thin yttria sta-bilized zirconia ([YSZ] electrolyte wafers with Ni/YSZ cermet anodes) provided by Ceramatec (Salt Lake City, UT) and agitated by a flowing CO2 stream produced a peak power density in excess of 140 mW/cm2, which deteriorated in time due to sulfur interaction with the Ni anode. Similar experiments using fluidized coal in flowing He gas with other button cells gave peak power densities in excess of 40 mW/cm2, which also decayed in time. Again, benchmarking tests on these same button cells using gaseous fuels only gave comparable power densities. These results pointed to the importance of the microstructure, stability, and catalycity of the anode and its impact on cell performance.
In all cases, gas analyses of the reaction products verified oxygen balance around the cell, and indicated that all oxygen, supplied electro-chemically through the solid electrolyte into the solid fuel bed, is ac-counted for in the form of CO and CO2 in the flue stream. These prelimi-nary results demonstrated for the first time that one can electrochemically convert solid carbonaceous fuels into electricity in a single step inside a fluidized bed reactor.
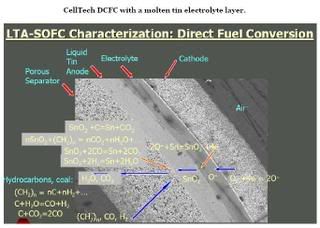
Celltech DCFC
CellTech Power is developing a technology that uses a liquid tin anode in a solid oxide fuel cell. This system oxidizes molten tin (Sn) to tin oxides (such as SnO2) in the anode layer by oxygen ions produced in the cathode. The ions transit a typical Yttria-stabilized Zirconia (YSZ) electro-lyte to reach the anode such that electrons are released. Electricity can be produced directly by oxidizing Sn like a battery.
The SnO2 can be reduced back to Sn by carbon-containing solids or any reducing gases consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur that enters the anode. During the Sn regeneration, the device operates like a fuel cell. The Sn anode is not poisoned by sulfur. With a cell open circuit voltage (OCV) of 0.8V, the CO/CO2 ratio is 0.2 in the anode effluent gas. Maintaining cell voltage (OCV) above 0.8V keeps the dissolved SnO2 concentration in the molten Sn at a level where precipitation of the oxide does not occur. This means that the CO-containing gaseous effluent that leaves the cell must be oxi-dized to complete the conversion of CO to CO2.
Several years ago, with $15 million raised from venture capital and private sources, CellTech built two 1 kW Gen 2 units fueled by natural gas, which operated for more than 2000 hrs continuously. In those Gen 2 units, the natural gas was conditioned to a stream also containing CO and hydrogen and fed to the Sn anode. During 2005-2006, with Defense Advanced Re-search Projects Agency (DARPA) funding, CellTech developed Gen 3.0 cells and stacks allowing direct conversion of waste packaging materials and JP-8 into electricity. Before 2005, the key limitations of this system had been low power density (with levels of 40 mW/cm2 with hydrogen fuel and 20 mW/cm2 with carbon/JP-8 fuel) and difficulty in manufacturing. These power densities had been deemed too low for portable and mobile power generation. With support from DARPA/Army recently in place, CellTech is developing a Gen 3.1 (2007) cell architecture for direct JP-8 conversion with improved power density. They have modified the porous media to allow higher mass transfer rates of heavy fuel molecules flowing to the anode and are developing a high electrical conductance tubular cathode.
In 2006, CellTech demonstrated power densities of 160 mW/cm2 for hydrogen and 80 mW/cm2 for JP-8. The Gen 3.1 design is expected to provide approximately four times reduction in weight and volume over the previous Gen 3.0. Gen 3.1 is projected to become competitive for number of portable and mobile applications such as military field battery chargers. The mid-term power density target for direct JP-8 conversion is 200 mW/cm2 (2008-2010); at this level the direct JP-8 conversion liquid Sn system becomes a formidable competitor for kilowatt or sub-kilowatt applications.
CellTech Power has several concepts of how to generate power from coal with this system, but has not completed a detailed flowsheet analysis. One approach involves feeding coal to a molten Sn bath anode to reduce SnO2 to Sn, then transferring the molten Sn to the cell arrays for oxidation to SnO2 and power production. Another concept is to use a fluidized bed of coal to take advantage of volatiles in coal, in which carbon in the coal is reacted with hot recycled CO2 and water to produce a CO-rich gas, which is then fed to the cell array to produce power.
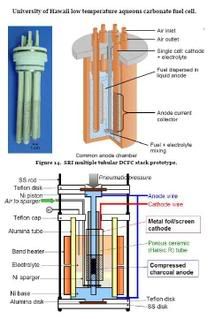
University of Hawaii's DCFC, designed for use with charcoal
Charcoal has been used as the feedstock for a low temperature aqueous carbonate fuel cell that has operated as high as 245 °C. At this temperature the cell offered an open circuit voltage of 0.57 V and a short circuit current of 43.6 mA/cm2. At 220 °C, the power density was 6.3 mW/cm2. One possible explanation for the relatively low open circuit voltages resulted from the formation of carbon oxides on the anode that were accompanied by the release of CO2.
Thermodynamically, oxygen reduction at the cathode is more favorable at temperatures below 200 °C, however, improved anode performance could result from a higher temperature that could combust the carbon oxides ac-cumulated on the bicarbon anode material. Therefore, performance could be markedly improved if a split cell could be developed in which the cathode could be operated at below 200 °C and the anode at above 240 °C.
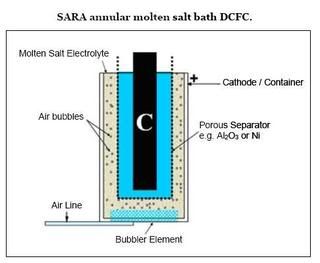
SARA's DCFC
SARA has evolved a new concept that uses different salts in two chambers separated by a porous separator plate. The cathode chamber contains molten potassium (KOH) or sodium hy-droxide (NaOH). Better results have been obtained with KOH. Moist air is bubbled into this chamber where the oxygen picks up electrons, resulting in the formation of OH- ions, which then transport through the separator membrane to enter the anode chamber. A basket of solid fuel particles is suspended in molten metal carbonates in the anode chamber. The OH- ions react with the solid fuel to produce CO3-2 ions and electrons. The CO3-2 ions also react with the coal to produce CO2 and electrons.
SARA recently observed that the electrolyte was stable over the course of a 500-hr experiment. A stackable design concept has been developed. They stated that the major challenges are the separator material and design, corrosion, and operating temperature. Power density numbers for this system are difficult to compare to the other systems reviewed because those numbers are based on the area of the separator rather an anode or cathode area.
Finally, one company, SRI has developed a concept based on feeding coal (as well as other carbon sources such as tar, biomass and waste paper/plastic) as a carbon rich solid fuel to a flowing molten salt, such as alkali metal carbonates. That mixture forms an electrically conducting anode when the carbon concentration reaches a value between 30 and 40 percent. Air is fed to a conventional SOFC cathode (typically strontium-doped lanthanum manganite [LSM]) which provides the oxygen ions that migrate through a solid oxide electrolyte (typically YSZ) and react with the solid fuel to produce electricity and CO2. SRI currently is operating a batch system which has up to six cathode/electrolyte tubes inserted into a single molten salt bath. Three types of tubes are being used: simplified, sub-scale, and full-scale
For the experiments conducted to date, the solid fuel is mixed with salt powder and the dry mixture is then dropped into the apparatus, which is then heated to its operating temperature of 800-950 °C. Power densities of approximately 300 mW/cm2 have been achieved. Lifetimes in excess of 1200 hours have also been demonstrated. A design for a 40 kW power system has been completed.
Integrating biomass pyrolysis and electricity production
As outlined above, there are several techniques for the production of a solid, carbon-rich, particulate fuel from various carbon-rich biomass and organic waste resources. These processes themselves generate heat and gases that can be utilized for the production of power. The question now is whether to integrate this operation with the DCFC's operation into a continuously operating system or to disperse the fuel preparation and electricity production functions to separate locations.
In the integrated case, it is necessary to transport the fuel to a central location and then transmit and distribute electricity from that location to a variety of users. The integrated approach offers an opportunity for maximum energy efficiency as a result of integration between the fuel preparation and fuel consumption operations as well as the opportunity to use that waste energy (thermal and methane) in other buildings or processing plants that could be co-located with the integrated unit.
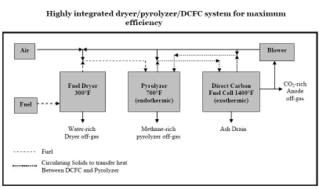
Figure 2 (click to enlarge) shows a system with the potential for the highest energy efficiency and best opportunity for energy integration with co-located facilities. In this concept, feed material is dried at about 149 °C to drive water off from the wet biomass/MSW feed material. The dried feed is then pyrolized at 371 °C to drive off methane and carbon dioxide and produce char which is fed to the DCFC. Hot, CO2-rich anode product gas is recycled from the DCFC to the fuel dryer and pyrolyzer to provide the heat energy needed for those operations.
Excess energy in the pyrolyzer waste gas and in the CO2 rich anode-off gas can be used for steam generation and perhaps used in co-located energy consuming facilities.
Overall process efficiency
In a recent overview of DCFC technologies, researchers at the ERDC-CERL Fuel Cell Program of the U.S. Army Corps of Engineers undertook a basic calculation of the overall efficiency of the process to go from raw carbon-rich, biomass to electricity. Since the work of all the teams involved in developing DCFC technology is at very early stages of development, it is not likely that a fully integrated stack and fuel cell power plant will achieve the maximum theoretical efficiency of 80 percent that occurs in a single cell.
The researchers therefor assumed that the efficiency of converting the chemical energy (Higher Heating Value or HHV basis) in the dry carbon in the fuel to AC electricity in the DCFC is 65 percent. (Typical pulverized coal power plants convert the chemical energy in coal (on the same HHV basis) to electricity at 30–35 percent efficiency.)
Wood received in a power plant is assumed to contain 45 percent moisture. The typical carbon composition of dry-wood is assumed to be 50 percent. The heat of combustion of carbon when CO2 is the only product is 14,087 Btu/pound. Therefore the amount of pure carbon required to produce 1 MWH of electricity is 372 lb. If dry wood contains 50 percent carbon, then 372 lb of carbon is contained in 744 lb of dry wood.
Assuming that wet wood contains 45 percent moisture, then the amount of wet wood required for 168.7kg (372 lb) of carbon or 337.5kg (744 lb) of dry wood is 613.7kg (1353 lb). It follows that the amount of water contained in the wet wood is 276.2kg (609 lb). Drying wood by evaporation requires approximately 1000 Btu of heat per pound of water evaporated. Removing 276.2kg (609 lb) of water from wet wood requires 642.5MJ (609,000 Btu) of energy.
Converting 168.7kg (372 lb) of carbon into 1 MWH of electricity in a DCFC liberates 1,939 MJ (1,838,000 Btu) of waste heat in the fuel cell. The amount of energy contained in 1 MWH of electricity is 3,599,851Kj, from which it follows that the waste heat available is 1,939,192.9 kJ/MWH.
To maintain the fuel cell at its constant operating temperature of approxi-mately 760 °C (1400 °F), this waste heat must be removed from the DCFC system both by heating up the reactants that are fed to it, and cooling and recycling the gas product from the cell.
The waste heat available of 1,939 MJ/MWH (1,838,000 Btu/MWH) is far more than the 642 MJ/MWH (609,000Btu/MWH) required for wet wood drying. It can therefore be used to supply the heat needed for evaporation of the water from the wet wood.
The pyrolysis step also liberates a significant amount of methane. A reasonable assumption is that 10 percent of the mass of dry wood fed to a pyrolysis reactor will be produced as methane. Therefore, 613.7kg (1353 lb) of wet wood containing 744 lb of dry wood will yield about 33.7kg (74.4 lb) of methane. This amount of methane contains about 2,110 MJ (2,000,000 Btu’s) of energy, which could be used for drying or pyrolysis, or which could be exported for external uses.
Capturing CO2: green extreme
Countries with extensive coal reserves will continue to use coal as their primary source of electricity for many years to come. Biomass will be co-fired or used in dedicated power plants more often, but coal is set to remain the most widely used fuel. However, today's coal-fired power plants convert coal into electricity with relatively low efficiency, often not higher than 30 to 35 percent. In addition, coal is a source of toxic emissions, greenhouse gases and heavy metal pollutants when used in traditional combustion power plants. For coal dependent countries to enter a more environmentally sustainable and economically feasible way, a clean, efficient and direct process to convert coal into electrical energy is needed. This is where the DCFC can play a major role.
The CO2 from conventional fossil fuel power plants can be captured via a range of processes - pre-combustion, oxyfuel or post-combustion capture - but separating the gas from other flue or process gases is complex, energy demanding and requires expensive membranes or adsorption technologies. Nonetheless, large funds are being poured into such carbon capture and storage (CCS) research projects.
DCFCs have the major advantage that the CO2 stream they generate is extremely pure, allowing the climate destructive gas to be captured with ease. Legislation allowing CCS and a carbon price would give DCFCs a competitive advantage because they significantly lower the CO2 capture cost. If carbon-dioxide-capturing costs are included, DCFC system costs are estimated to be up to 50 percent less than those of current fossil fuel plants.
What is more, the fact that DCFCs can operate on the carbon contained in biomass, allows for the development of the most radically green type of energy system imaginable: one that results in negative emissions.
This is possible because as biomass grows it sequesters atmospheric CO2. When this gas is captured at the DCFC and then stored in geological formations such as depleted oil and gas fields or saline aquifers, the biomass actively removed CO2 from the atmosphere. Instead of being merely carbon-neutral (as other types of renewable energy are), the energy thus obtained would be carbon-negative.
 Biopact readers are aware of research which shows that when biomass is burned in integrated gasification combined cycle (IGCC) plants, with the CO2 captured and stored, that negative emissions as high as minus 1000 grams of CO2 per KWh can be obtained. Solar photovoltaic (+100grams), wind (+30grams), non-CCS biomass (+30grams) and nuclear (+10-20 grams) are all carbon positive (graph, click to enlarge). Biomass burned in an IGCC coupled to CCS goes way beyond that and removes historic CO2 from the atmosphere. It is thus by far the most radical tool in the climate fight.
Biopact readers are aware of research which shows that when biomass is burned in integrated gasification combined cycle (IGCC) plants, with the CO2 captured and stored, that negative emissions as high as minus 1000 grams of CO2 per KWh can be obtained. Solar photovoltaic (+100grams), wind (+30grams), non-CCS biomass (+30grams) and nuclear (+10-20 grams) are all carbon positive (graph, click to enlarge). Biomass burned in an IGCC coupled to CCS goes way beyond that and removes historic CO2 from the atmosphere. It is thus by far the most radical tool in the climate fight.However, IGCCs are only moderately efficient, because they rely on the combustion of fuels; a considerable amount of the energy generated would be needed to capture the CO2, thus lowering the overall systems efficiency of IGCCs + CCS. DCFCs in contrast are up to twice as efficient and would thus allow the capture of CO2 in a far more efficient manner still. There are no calculations showing the overall efficiency of capturing CO2 from biomass by using part of the electricity generated in a DCFC, but it can be safely assumed that the process would be far more efficient than the same operation performed in a IGCC.
In short, the confluence of different factors in operating direct carbon fuel cells - the purity of the CO2 stream and the efficiency of the electricity generating process - makes it possible to imagine what is possibly the cleanest and greenest form of electricity production imaginable: efficiently generated negative emissions energy. Efficient DCFCs could help clean up the atmosphere and help us prevent climate change in the most drastic way.
References:
A large number of older, but key articles can be found online at the National Technology Energy Library: Direct Carbon Fuel Cell Workshop.
University of Hawaii; Hawaii Natural Energy Institute - Renewable Resources Research Laboratory: Biocarbon Fuel Cells.
Ronald H. Wolk, Scott Lux, Stacy Gelber, and Franklin H. Holcomb, Direct Carbon Fuel Cells: Converting Waste to Electricity [*.pdf], U.S. Army Corps of Engineers: ERDC-CERL Fuel Cell Program, September 2007
Antal, Michael J, Performance of a First Generation Aqueous Alkaline Biocarbon Fuel Cell, Ind. Eng. Chem. Res., 46 (3), 734 -744, 2007. DOI: 10.1021/ie061202s S0888-5885(06)01202-4
Cao, D., Y. Sun, G. Wang, Direct Carbon Fuel Cell: Fundamentals and Recent Developments, Journal of Power Sources, vol 167, No. 2, pp 250-257, May 15, 2007.
Hackett, Gregory A., John W. Zondlo, Robert Svensson. “Evaluation of Carbon Materials for Use in a Direct Carbon Fuel Cell,” Journal of Power Sources, vol 168, No. 1, pp 111-118, May 25, 2007.
Heydorn, Barbara, and Steven Crouch-Baker, “Direct Carbon Conversion: Progressions of Power,” The Fuel Cell Review, 2006.
Lawrence Livermore National Laboratory: Direct Carbon Fuel Cells.
SRI International: SRI International Presents Novel Direct Carbon Fuel Cell Technology at Industry Event - November, 11, 2005
SARA: Direct Carbon Fuel Cell.
Biopact: Back to black: hydrothermal carbonisation of biomass to clean up CO2 emissions from the past - May 26, 2007
 --------------
--------------
 Austrian bioenergy group Cycleenergy acquired controlling interest in Greenpower Projektentwicklungs GmbH, expanding its biomass operational portfolio by 16 MW to a total of 22 MW. In the transaction Cycleenergy took over 51% of the company and thereby formed a joint venture with Porr Infrastruktur GmbH, a subsidiary of Austrian construction company Porr AG. Greenpower operates two wood chip CHP facilities in Upper and Lower Austria, each with an electric capacity of 2 MW. The plants have been in operation since the middle of last year and consume more than 30,000 tonnes of wood chips and are expected to generate over €5 million in additional revenue.
Austrian bioenergy group Cycleenergy acquired controlling interest in Greenpower Projektentwicklungs GmbH, expanding its biomass operational portfolio by 16 MW to a total of 22 MW. In the transaction Cycleenergy took over 51% of the company and thereby formed a joint venture with Porr Infrastruktur GmbH, a subsidiary of Austrian construction company Porr AG. Greenpower operates two wood chip CHP facilities in Upper and Lower Austria, each with an electric capacity of 2 MW. The plants have been in operation since the middle of last year and consume more than 30,000 tonnes of wood chips and are expected to generate over €5 million in additional revenue.








0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home