Ceramic tubes could cut greenhouse gas emissions from power stations

The material, known as LSCF, has the remarkable property of being able to filter oxygen out of the air. By burning fuel in pure oxygen, it is possible to produce a stream of almost pure carbon dioxide, which has commercial potential for reprocessing into useful chemicals.
LSCF is not a brand new material - it was originally developed for fuel cell technology - but the engineers have developed it for potential use in reducing emissions for gas-fired power stations and possibly coal and oil-fired electricity generation as well.
The cheapest way to dispose of waste carbon dioxide from combustion is to release it into the atmosphere. We have been doing this since humans first discovered how to make fire. The technology we have developed may provide a viable alternative, although whether it is economical to introduce it will depend largely upon the carbon credit system that Governments operate in the future. - Professor Ian Metcalfe, School of Chemical Engineering and Advanced Materials at Newcastle UniversityCapturing CO2
Conventional gas-fired power stations burn methane in a stream of air, producing a mixture of nitrogen and greenhouse gases including carbon dioxide and nitrogen oxides, which are emitted into the atmosphere. Separating the gases is not practical because of the high cost and large amount of energy needed to do so.
However, the LSCF tubes would allow only the oxygen component of air to reach the methane gas, resulting in the production of almost pure carbon dioxide and steam, which can easily be separated by condensing out the steam as water.
The resulting stream of carbon dioxide could be piped to a processing plant for conversion into chemicals such as methanol, a useful industrial fuel and solvent. Alternatively it could be sequestered.
The new combustion process has been developed and tested in the laboratory by Professor Ian Metcalfe, Dr Alan Thursfield and colleagues in the School of Chemical Engineering and Advanced Materials at Newcastle University, in collaboration with Dr Kang Li in the Chemical Engineering Department at Imperial College London. The research has been funded by the Engineering and Physical Sciences Research Council (EPSRC).
Details of the research and development project are published on 3 August 2007 simultaneously in two technical publications - Materials World and The Chemical Engineer. A series of research papers have also been published in academic journals as the project has developed.
How the tubes work
The LSCF tubes look like small, stiff, drinking straws and are permeable to oxygen ions — individual atoms carrying an electrical charge. Crucially, LSCF is also resistant to corrosion or decomposition at typical power station operating temperatures of around 800C:
 energy :: sustainability :: biomass :: bioenergy :: biofuels :: greenhouse gas emissions :: carbon dioxide :: combustion :: biomethane :: carbon-negative ::
energy :: sustainability :: biomass :: bioenergy :: biofuels :: greenhouse gas emissions :: carbon dioxide :: combustion :: biomethane :: carbon-negative :: When air is blown around the outside of the tubes, oxygen is able to pass through the wall of the tube to the inside, where it combusts with methane gas that is being pumped through the centre of the tubes.
The oxygen-depleted air, which consists mainly of nitrogen, can be returned to the atmosphere with no harmful effects on the environment, while the carbon dioxide can be collected separately from the inside of the tubes after combustion.
An alternative would be to control the flow of air and methane so that only partial combustion took place. This would result in a flow of 'synthesis gas', a mixture of carbon monoxide and hydrogen, which can easily be converted into a variety of useful hydrocarbon chemicals.
The tubes of LSCF, which stands for Lanthanum-Strontium-Cobalt-Ferric Oxide, have been tested successfully in the laboratory and the design is attracting interest from the energy industry. The Newcastle team is now carrying out further tests on the durability of the tubes to confirm their initial findings that they could withstand the conditions inside a power station combustion chamber for a reasonable length of time.
Although it has not yet been attempted, it should be possible to assemble a power station combustion chamber from a large number of the tubes, with space between them for air to circulate.
In theory the technology could also be applied to coal and oil-fired power stations, provided that the solid and liquid fuels were first converted into gas. This operation is simple in theory but would add to the cost and complexity of running a power station.
Government statistics suggest that the UK energy industry produces over 200 million tonnes of carbon dioxide per year, which is more than one-third of the country's total carbon dioxide emissions.
LSCF is a relatively new material and over the past ten years or so been the subject of research in many countries, mainly into its potential use as a cathode in fuel cells.
Picture: Professor Ian Metcalfe with the ceramic tubes in his laboratory at Newcastle University, England.
References:
Newcastle University: Ceramic membrane could cut greenhouse gas emissions from power stations - August 3, 2007.
Article continues
 --------------
--------------
 Taiwan's Feng Chia University has succeeded in boosting the production of hydrogen from biomass to 15 liters per hour, one of the world's highest biohydrogen production rates, a researcher at the university said Friday. The research team managed to produce hydrogen and carbon dioxide (which can be captured and stored) from the fermentation of different strains of anaerobes in a sugar cane-based liquefied mixture. The highest yield was obtained by the Clostridium bacterium.
Taiwan's Feng Chia University has succeeded in boosting the production of hydrogen from biomass to 15 liters per hour, one of the world's highest biohydrogen production rates, a researcher at the university said Friday. The research team managed to produce hydrogen and carbon dioxide (which can be captured and stored) from the fermentation of different strains of anaerobes in a sugar cane-based liquefied mixture. The highest yield was obtained by the Clostridium bacterium.



 Biohydrogen, biomethane and biomass based syngas may soon be burned in an experimental gas turbine simulator equipped with an ultralow-emissions combustion technology to yield extremely clean renewable electricity. Called LSI, the technique has been tested successfully using pure hydrogen as a fuel – a milestone that indicates a potential to help eliminate millions of tons of carbon dioxide and thousands of tons of NOx from power plants each year.
Biohydrogen, biomethane and biomass based syngas may soon be burned in an experimental gas turbine simulator equipped with an ultralow-emissions combustion technology to yield extremely clean renewable electricity. Called LSI, the technique has been tested successfully using pure hydrogen as a fuel – a milestone that indicates a potential to help eliminate millions of tons of carbon dioxide and thousands of tons of NOx from power plants each year.


 Luiz Inacio Lula Da Silva is the president not only of Brazil, but of the largest black community living outside the African continent. The Latin American nation has profound cultural and social links with the continent at the other side of the Atlantic that go back centuries. President Lula is undoubtedly the first Brazilian leader to revive these relations and to see them as a great opportunity for cooperation. Under his time in office, Brazil for the first time became a net donor of development assistance to Africa.
Luiz Inacio Lula Da Silva is the president not only of Brazil, but of the largest black community living outside the African continent. The Latin American nation has profound cultural and social links with the continent at the other side of the Atlantic that go back centuries. President Lula is undoubtedly the first Brazilian leader to revive these relations and to see them as a great opportunity for cooperation. Under his time in office, Brazil for the first time became a net donor of development assistance to Africa. The
The  If it exploits its enormous biofuel potential fully and effectively, Africa can defeat poverty and achieve the Millennium Development Goals (MDGs), a team of experts
If it exploits its enormous biofuel potential fully and effectively, Africa can defeat poverty and achieve the Millennium Development Goals (MDGs), a team of experts 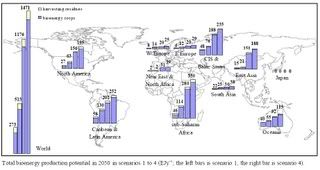
 Indonesia is set to become one of the leading biofuel producers, with a variety of crops being promoted - from sugarcane and cassava, to jatropha and palm oil. Biofuels and bioenergy offer the country the opportunity to substantially reduce reliance on extremely expensive oil and to bring millions of jobs to the poor. Technically this may be possible, but success will depend on carefully crafted policies and legislation (
Indonesia is set to become one of the leading biofuel producers, with a variety of crops being promoted - from sugarcane and cassava, to jatropha and palm oil. Biofuels and bioenergy offer the country the opportunity to substantially reduce reliance on extremely expensive oil and to bring millions of jobs to the poor. Technically this may be possible, but success will depend on carefully crafted policies and legislation (
 The EU's Emission Trading Scheme is facing a revolt by Eastern European countries who claim their carbon allowances, set by the Commission, are too low. Six member states are considering taking legal action against the executive branch of the Union. If they win, the European carbon market and the scheme to make it work would end up in ruins.
The EU's Emission Trading Scheme is facing a revolt by Eastern European countries who claim their carbon allowances, set by the Commission, are too low. Six member states are considering taking legal action against the executive branch of the Union. If they win, the European carbon market and the scheme to make it work would end up in ruins. Crops are facing a growing number of problems: too much water, too little sunlight, droughts, a changing atmosphere... In short, they are suffering from all kinds of stress. Climate change may make things worse by transforming certain regions into becoming high stress environments, whereas in others conditions will change in a more beneficial way, but requiring plants to adapt just as dramatically. In order to develop
Crops are facing a growing number of problems: too much water, too little sunlight, droughts, a changing atmosphere... In short, they are suffering from all kinds of stress. Climate change may make things worse by transforming certain regions into becoming high stress environments, whereas in others conditions will change in a more beneficial way, but requiring plants to adapt just as dramatically. In order to develop  The Automotive X PRIZE (AXP), a competition designed to inspire a new generation of viable, super-efficient vehicles to help break America's addiction to oil and stem the effects of climate change,
The Automotive X PRIZE (AXP), a competition designed to inspire a new generation of viable, super-efficient vehicles to help break America's addiction to oil and stem the effects of climate change, 
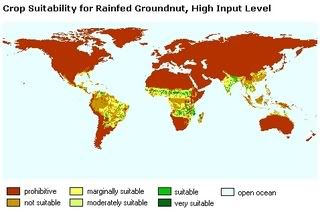
 Gull New Zealand
Gull New Zealand  Fonterra's Edgecumbe dairy factory in the Bay of Plenty successfully tested petrol mixed with 10 per cent ethanol in a 1.8-litre car, in a blend approved by the Environmental Risk Management Authority. The Edgecumbe plant produces 30,000 litres of ethanol a day and over five million litres in a dairy season. Fonterra also produces ethanol at other plants, including Reporoa and Tirau, for use in industrial cleansers, vodka and gin:
Fonterra's Edgecumbe dairy factory in the Bay of Plenty successfully tested petrol mixed with 10 per cent ethanol in a 1.8-litre car, in a blend approved by the Environmental Risk Management Authority. The Edgecumbe plant produces 30,000 litres of ethanol a day and over five million litres in a dairy season. Fonterra also produces ethanol at other plants, including Reporoa and Tirau, for use in industrial cleansers, vodka and gin: Is it economically viable and sustainable to produce hydrogen outside the EU from clean and renewable sources (biomass, wind, solar, hydro, geothermal), and then import it over very long distances to consumers in the Union? The answer is yes, according to
Is it economically viable and sustainable to produce hydrogen outside the EU from clean and renewable sources (biomass, wind, solar, hydro, geothermal), and then import it over very long distances to consumers in the Union? The answer is yes, according to 

 By-products from biofuels could help beef up livestock feed in the future, according to a study completed by environmental research group
By-products from biofuels could help beef up livestock feed in the future, according to a study completed by environmental research group 
 South Africa is threading carefully towards a biofueled future. More thorough policy work and consultation are underway to ensure that the sector benefits smallholders first and that regulation is based on a realistic assessment of South Africa's (relatively small) technical potential. The country's Science and Technology Minister, Mosibudi Mangena, said legislation will not be finalized by the end of this year.
South Africa is threading carefully towards a biofueled future. More thorough policy work and consultation are underway to ensure that the sector benefits smallholders first and that regulation is based on a realistic assessment of South Africa's (relatively small) technical potential. The country's Science and Technology Minister, Mosibudi Mangena, said legislation will not be finalized by the end of this year.


 According to Credit Suisse Research, Malaysian company
According to Credit Suisse Research, Malaysian company 











Saturday, August 04, 2007
Danish researchers look at seaweed for biofuels
The aquatic plant is a large, fast growing green algae that can be found near shores (profile at the AlgaeBase; image, click to enlarge). It thrives in nutrient-rich zones, especially there where water is contaminated by nitrogen runoff from agriculture. Interestingly, its sugar content is relatively high, making it a potential feedstock for cellulosic ethanol production.
Michael Bo Rasmussen of the National Institute of Environmental Research at the University of Aarhus has already carried out two tests with the algae and thinks harvesting them as a biomass source might make sense. The species grows fast, doubling its biomass every three to four days. Rasmussen estimates the theoretical yearly yield to be between 200 and 500 tons of wet biomass on a 'hectare' basis (even though comparisons with terrestrial plants are difficult). Denmark's total potential would be an annual production of around 80,000 to 100,000 tons. Importantly, the algae doesn't need fresh water to grow and it occurs near shores, making it accessible. The seaweed could be harvested in its wild form, and thus contribute to re-oxygenating zones that have been invaded by the algae.
Large scale cultivation
Like Japanese researchers, the Danish scientists are thinking of cultivating the algae on a large scale. Traditional seaweed cultivation techniques, refined in Japan, could be modernized and applied to a modern aquacultural biomass industry. Ulva lactuca thrives when fed with liquid fertilizer and carbon dioxide, the greenhouse gas. Denmark's economy generates an excess of both these nutrients. Rasmussen estimates that an optimal production process based on feeding the algae the right amount of fertilizer and CO2, could yield up to 500 tons of biomass per hectare. But for the time being, such a high-tech form of aquaculture would be prohibitively expensive, the researcher says:
Rasmussen's project is one of the proposals selected for funding by the Aarhus Research Foundation, which is freeing up 48 million kroner (€6.4/US$8.8 million) for 16 different projects over the 2007-2011 period.
The idea of harvesting algae from the open ocean keeps popping up each time oil prices reach records. In the 1970s, several similar ideas were launched and received modest funding, both in the U.S., Japan and the EU. Scientists can now pick up on the research of their older collegues. Recently, a company that used to work on micro-algae production in closed photobioreactors decided to do just that and started looking at harvesting biomass from algae blooms found in the open ocean (previous post).
Some of these aquacultural projects may make sense over the ultra-long term provided major R&D breakthroughs are made. However, ideas like growing algae in closed photobioreactors are not feasible (more here) because uncompetitive; likewise, growing the micro-organisms in open ponds requires serious advances in biotechnology to bring costs down by at least a factor of 20.
Picture: Ulva lactuca Linnaeus. Photographer: Katrin Österlund © Katrin Österlund. Oliveira, E., Österlund, K. & Mtolera, M.S.P. (2005). Marine Plants of Tanzania. A field guide to the seaweeds and seagrasses. pp. 267. Stockholm: Botany Department, Stockholm University. Credit: AlgaeBase.
References:
Futura-Sciences: Une idée danoise : le biocarburant à base de laitue de mer - July 25, 2007.
University of Aarhus: Stort potentiale i biobrændstof fra havet - June 222, 2007.
AlgaeBase: Ulva lactuca Linnaeus profile.
Biopact: Scientist skeptical of algae-to-biofuels potential - interview - July 18, 2007
Biopact: Harvesting algae blooms from the open ocean - March 01, 2007
Article continues
posted by Biopact team at 11:14 PM 0 comments links to this post
