Researchers study effectiveness of glycerin as cattle feed
 Global biodiesel production is increasing rapidly. The consequence is that a byproduct obtained from making the biofuel, glycerin (glycerol), is beginning to flood the market. As vegetable oils are transesterified into biodiesel, some 10% of the resulting product consists of glycerin. In short, it is an important co-product. Finding new applications for it is crucial to make biodiesel production more competitive with fossil fuels. Researchers are making progress: so far they found that glycerin can either be used as a biofuel in itself, as a feedstock for the production of biogas, or for green specialty chemicals (such as propylene glycol). Other scientists tested glycerin as a poultry feed and found positive results. A recent EU-study showed that if used as a feed component, the massive influx of the byproduct may even result in lower meat prices for consumers.
Global biodiesel production is increasing rapidly. The consequence is that a byproduct obtained from making the biofuel, glycerin (glycerol), is beginning to flood the market. As vegetable oils are transesterified into biodiesel, some 10% of the resulting product consists of glycerin. In short, it is an important co-product. Finding new applications for it is crucial to make biodiesel production more competitive with fossil fuels. Researchers are making progress: so far they found that glycerin can either be used as a biofuel in itself, as a feedstock for the production of biogas, or for green specialty chemicals (such as propylene glycol). Other scientists tested glycerin as a poultry feed and found positive results. A recent EU-study showed that if used as a feed component, the massive influx of the byproduct may even result in lower meat prices for consumers.Along the same lines, researchers from the University of Missouri-Columbia are now studying whether glycerin can be used to feed cattle. In a study that began this month, Monty Kerley, professor of ruminant nutrition in the College of Agriculture, Food and Natural Resources, is examining the effectiveness of glycerin as component in cattle diets. Through November, the MU researcher will monitor the growth habits of 60 calves from various breeds to determine if the leftovers provide a healthy main course to cattle. The study has two main priorities: first, to determine if glycerin has a positive or negative effect on calves' growth performance, and second, to assess its impact, if any, on meat quality.
The cows have been separated into groups of three, each consuming differing amounts of glycerin during their daily diet. The amounts are 0, 5, 10 and 20 percent. In addition to monitoring feeding limits and growth patterns, Kerley also is analyzing how cattle metabolize the varying amounts of glycerin. Unlike the dry feeds they are accustomed to eating, Kerley said the glycerin is liquid based and comes mostly from the processing of soybean oil. He also said it meets stringent Food and Drug Administration regulations:
 bioenergy :: biofuels :: energy :: sustainability :: biodiesel :: vegetable oil :: glycerin :: nutrition :: cattle feed ::
bioenergy :: biofuels :: energy :: sustainability :: biodiesel :: vegetable oil :: glycerin :: nutrition :: cattle feed :: "We're really looking at the energy value and how it compares to corn," Kerley said. "When the animal consumes glycerin, it's absorbed, and the glycerin is used to make glucose. Actually, it's like feeding sugar to a cow. Because it's liquid, there are two things we worry about - one, how much can be used in the diet before it changes the form of the diet; and two, is there a limit to how much glycerin can be processed by the animal? We'll feed it to them for a period of 160 to 180 days."
Kerley said developing usages for glycerin necessitates this type of research. In recent years, academic scientists and private-sector companies have been racing to find solutions and applications for the byproduct. An alternative food source for cattle is but one possibility. However, it's likely only a short-term option for the cattle industry.
"We probably have a three- to five-year window to use this for animal feed at a reduced cost," Kerley said. "This glycerin is a wonderful starting compound for building other compounds that can be applied to numerous industrial purposes. After three to five years, you'll see industrial applications utilizing this glycerin, and that may price it out of the animal feed industry."
He said economics are another factor because glycerin is currently less expensive than corn, which is most commonly used as cattle feed. Glycerin is about 4 cents per pound; corn costs around 8 cents a pound.
"Originally, the biodiesel plants were concerned with just getting rid of this material, but data shows that glycerin has energy feed value equal to corn," Kerley said. "If you can get glycerin for less than corn, that's obviously a sizeable savings."
Image: biodiesel production is achieved by transesterifying vegetable oils. Glycerin settling at the bottom of a batch of biodiesel. Biodiesel production results in a fraction of 10% glycerol, making it an important byproduct.
Article continues
 --------------
--------------
 Taiwan's Feng Chia University has succeeded in boosting the production of hydrogen from biomass to 15 liters per hour, one of the world's highest biohydrogen production rates, a researcher at the university said Friday. The research team managed to produce hydrogen and carbon dioxide (which can be captured and stored) from the fermentation of different strains of anaerobes in a sugar cane-based liquefied mixture. The highest yield was obtained by the Clostridium bacterium.
Taiwan's Feng Chia University has succeeded in boosting the production of hydrogen from biomass to 15 liters per hour, one of the world's highest biohydrogen production rates, a researcher at the university said Friday. The research team managed to produce hydrogen and carbon dioxide (which can be captured and stored) from the fermentation of different strains of anaerobes in a sugar cane-based liquefied mixture. The highest yield was obtained by the Clostridium bacterium.


 In 2005, professor Parente won the Blue Sky Award for his aircraft fuel project, "a kind of Nobel prize given by the UN for innovations in the field of renewable energies", he comments.
In 2005, professor Parente won the Blue Sky Award for his aircraft fuel project, "a kind of Nobel prize given by the UN for innovations in the field of renewable energies", he comments.
 Developing and designing innovative biorefinery concepts to make biomass-derived products cost-competitive with fossil fuels is the goal of the EU's newly launched
Developing and designing innovative biorefinery concepts to make biomass-derived products cost-competitive with fossil fuels is the goal of the EU's newly launched  A refreshing
A refreshing  Cuba is quietly modernizing its ethanol-producing facilities, despite Fidel Castro's repeated assertions that making more of the biofuel is a bad idea. Hypocrisy or not, the fact is simple: Cuba has the capacity to become a major ethanol producer, and it will not hesitate to take advantage of the opportunity. Earlier we reported on how the emerging biofuels market may revive the island's once thriving sugar industry (
Cuba is quietly modernizing its ethanol-producing facilities, despite Fidel Castro's repeated assertions that making more of the biofuel is a bad idea. Hypocrisy or not, the fact is simple: Cuba has the capacity to become a major ethanol producer, and it will not hesitate to take advantage of the opportunity. Earlier we reported on how the emerging biofuels market may revive the island's once thriving sugar industry (


 Estimated total costs of the piloting are €5 to 10/US$6.7 to 13.4 million. Pilot testing is expected to be finished by the end of 2008. The co-operation also covers the design and supply of a commercial scale biomass gasification plant. UPM announced in October 2006 that it will strongly increase its stake in second generation biodiesel in the next few years and prepares to become a significant producer of renewable biofuels. The main raw material used in UPM's biodiesel production will be wood based biomass:
Estimated total costs of the piloting are €5 to 10/US$6.7 to 13.4 million. Pilot testing is expected to be finished by the end of 2008. The co-operation also covers the design and supply of a commercial scale biomass gasification plant. UPM announced in October 2006 that it will strongly increase its stake in second generation biodiesel in the next few years and prepares to become a significant producer of renewable biofuels. The main raw material used in UPM's biodiesel production will be wood based biomass: The socio-economic situation in Nigeria's volatile delta region is tragic: the abundance of oil and gas has brougth immense wealth to a small corrupt elite and Western energy companies, whereas the vast mass of ordinary citizens has become impoverished. An economic crisis fueled by demographic changes (rapid population growth, urbanisation, the abandonment of agriculture) has triggered an enviromental crisis which makes things worse. Especially the younger generation of Delta inhabitants has become totally disillusioned and tilts towards using violence to express its frustration over this state of affairs.
The socio-economic situation in Nigeria's volatile delta region is tragic: the abundance of oil and gas has brougth immense wealth to a small corrupt elite and Western energy companies, whereas the vast mass of ordinary citizens has become impoverished. An economic crisis fueled by demographic changes (rapid population growth, urbanisation, the abandonment of agriculture) has triggered an enviromental crisis which makes things worse. Especially the younger generation of Delta inhabitants has become totally disillusioned and tilts towards using violence to express its frustration over this state of affairs.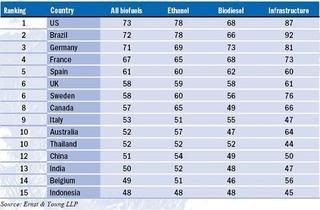
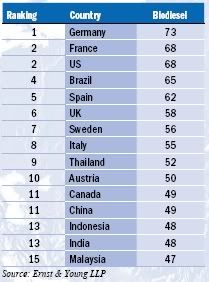 Germany tops the Biodiesel Index with a long history of governmental and financial support for the industry but its score would have been higher were it not for last year’s reduction in excise tax incentives, in favour of blending targets, leading to concerns that the market is reaching overcapacity.
Germany tops the Biodiesel Index with a long history of governmental and financial support for the industry but its score would have been higher were it not for last year’s reduction in excise tax incentives, in favour of blending targets, leading to concerns that the market is reaching overcapacity.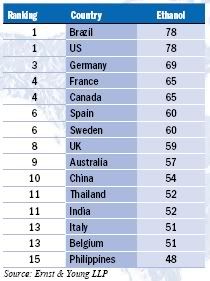 Brazil and the US are joint top of the Ethanol Index (
Brazil and the US are joint top of the Ethanol Index (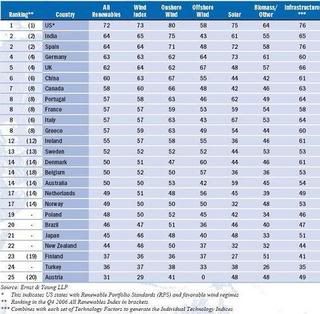
 To calculate the greenhouse gas balance of a biofuel, all the energy inputs of its entire production chain must be taken into account. If an ethanol plant uses coal or natural gas to power its operations (as is the case in the US and the EU), then the fuel will not be that clean. If however it uses renewable and climate friendly biomass (as is the case in Brazil where bagasse powers the refineries), then a much greener fuel can be obtained.
To calculate the greenhouse gas balance of a biofuel, all the energy inputs of its entire production chain must be taken into account. If an ethanol plant uses coal or natural gas to power its operations (as is the case in the US and the EU), then the fuel will not be that clean. If however it uses renewable and climate friendly biomass (as is the case in Brazil where bagasse powers the refineries), then a much greener fuel can be obtained. Manure from soil-surfaced pens may not always meet the minimum heating value on an as-received basis, Auvermann said. Feedyard operators will have to take some steps to improve it. The timeliness of collection and depth of scraping will be key to keeping dirt content below 60 percent and moisture content below 20 percent, he said. "Paving the pens with a crushed ash or a fly-ash material (from coal-fired power plants) will end up returning to you in the form of heating value – big time," Auvermann said.
Manure from soil-surfaced pens may not always meet the minimum heating value on an as-received basis, Auvermann said. Feedyard operators will have to take some steps to improve it. The timeliness of collection and depth of scraping will be key to keeping dirt content below 60 percent and moisture content below 20 percent, he said. "Paving the pens with a crushed ash or a fly-ash material (from coal-fired power plants) will end up returning to you in the form of heating value – big time," Auvermann said. In what is a breakthrough for the hydrogen economy, scientists from Virginia Tech, Oak Ridge National Laboratory (ORNL), and the University of Georgia
In what is a breakthrough for the hydrogen economy, scientists from Virginia Tech, Oak Ridge National Laboratory (ORNL), and the University of Georgia 

 The Philippines
The Philippines  Wild relatives of plants such as the potato and the peanut are at risk of extinction, threatening a valuable source of genes that are necessary to boost the ability of cultivated crops to resist pests and tolerate drought, according to a
Wild relatives of plants such as the potato and the peanut are at risk of extinction, threatening a valuable source of genes that are necessary to boost the ability of cultivated crops to resist pests and tolerate drought, according to a 
 In
In 
 Monthly ethanol imports to the U.S. in March fell 33% to 629,000 barrels in 14 shipments, according to preliminary
Monthly ethanol imports to the U.S. in March fell 33% to 629,000 barrels in 14 shipments, according to preliminary 



 T
T
 In order to diversify the portfolio of crops grown for the production of biomass and biofuels, scientists at the Texas A&M University's Agricultural Experiment Station (TAES) are breeding a drought tolerant sorghum that may yield between 37 and 50 tons of dry biomass per hectare (15 to 20 tons per acre).
In order to diversify the portfolio of crops grown for the production of biomass and biofuels, scientists at the Texas A&M University's Agricultural Experiment Station (TAES) are breeding a drought tolerant sorghum that may yield between 37 and 50 tons of dry biomass per hectare (15 to 20 tons per acre). At a time when the EU threatens with a
At a time when the EU threatens with a  Almost half of the world's population relies on fuel wood, charcoal, dung, or kerosene as fuels for cooking and heating. Most often, it is women and children who are burdened with the tasks of collecting the fuel and who prepare the food. Indoor smoke pollution from cooking on an open fire is a real killer in the kitchen. A recent analysis by the World Health Organisation shows around 1.5 million women and children die each year because of it (
Almost half of the world's population relies on fuel wood, charcoal, dung, or kerosene as fuels for cooking and heating. Most often, it is women and children who are burdened with the tasks of collecting the fuel and who prepare the food. Indoor smoke pollution from cooking on an open fire is a real killer in the kitchen. A recent analysis by the World Health Organisation shows around 1.5 million women and children die each year because of it (







Saturday, May 26, 2007
Back to black: hydrothermal carbonisation of biomass to clean up CO2 emissions from the past
Cleaning up the past: going carbon-negative
A growing number of scientists however is looking at ways to clean up our emissions from the past. In order to avert 'catastrophic' climate change, it has become desirable to invert the current development by sequestering the atmospheric CO2 of the past 200 years of industrialization. Some have suggested the rapid implementation of geo-engineering options, such as building vast forests of artificial trees that can suck CO2 out of the atmosphere and store it in geological formations (such as depleted coal or gas fields or saline aquifers). Such 'synthetic trees' would however be quite costly and will not deliver energy as they do their work (earlier post).
Gradually, a new, 'low-tech' geosequestration option is attracting the attention of more and more scientists. It is based on converting biomass into an inert form of bio-coal or charcoal, that can be stored in soils. Earlier we referred to carbon-negative energy systems that rely on gasification and biochar sequestration: biomass is gasified which results in a carbon monoxide and hydrogen rich gas that can be used for energy or transformed into ultra-clean synthetic biofuels via the Fischer-Tropsch process, whereas a fraction becomes bio-char that can be stored in soils (using a technique known as 'terra preta'). Similar techniques can be build around pyrolysis processes (earlier post). In such systems, soil fertility would be gradually enhanced, 'historic' CO2 would be sequestered and clean biofuels could be used to power our societies.
Only biomass can be used for the creation of such carbon-negative energy systems that clean up our emissions from the past. Other renewables are carbon-neutral at best, meaning they can only reduce future CO2 emissions - something many scientists think is not enough to avert dangerous climate change.
Maria-Magdalena Titirici, Arne Thomas and Markus Antonietti of the Department of Colloid Chemistry at the Max Planck Institute of Colloids and Interfaces, now describe a new, highly efficient though 'low-tech' way to use biomass as a tool to clean up past emissions. Their research appears in an open access article in the New Journal of Chemistry, in which they suggest creating "turbo-rainforests" based on fast-growing energy crops that are grown, turned into bio-coal via a process known as hydrothermal carbonization (HTC), and then stored into 'carbon landfills', while deriving energy from the process. The technique can be practised on an ultra-large scale, and can thus be described as a geo-engineering option - one that is actually technically and economically feasible.
Importantly, in contrast to other biomass carbonisation techniques that require dry biomass, the hydrothermal carbonisation process is a highly efficient 'wet' process that avoids complicated drying schemes and costly isolation procedures. The resulting carbonaceous materials also open a new field of chemistry, full of novel possibilities and challenges that may lead to the development of new (nano)materials:
Biomass as a carbon converter
The biggest carbon converter, with the highest efficiency to bind CO2 from the atmosphere, is certainly biomass, the scientists write. A rough estimate of terrestrial biomass growth amounts to 118 × 109 tons per year, when calculated as dry matter. Biomass, however, is just a short term, temporary carbon sink, as microbial decomposition liberates exactly the amount of CO2 formerly bound in the plant material:
To make biomass effective as a carbon sink, the carbon in it has to be fixed by low-tech operations. Coal formation is certainly one of the natural sinks that has been active in the past on the largest scale. Natural coalification of biomass takes place on a timescale of hundred million years. Due to its slowness, it is usually not considered in renewable energy exploitation schemes or as an active sink in CO2 cycles. Nevertheless, it is obvious that carbon fixation into coal is a lasting effort, as brown or black coal (on the contrary to peat) are obviously practically not biodegradable. The question of coalified carbon destabilization is, however, currently accessed in more detail. Sufficient condensation of the carbon scaffold is, in any case, mandatory for the purposes of carbon fixation.
It is therefore the purpose of this contribution to discuss the feasibility of turning coal formation into an active element of carbon sequestration schemes, simply by accelerating the underlying coalification processes by chemical means. The natural process of peat or coal formation is presumably not biological but chemical in its nature. As coaling is a rather elemental experiment, coals and tars have been made and used by mankind since the Stone Age, and one can find trials to imitate carbon formation from carbohydrates with faster chemical processes in the modern scientific literature. In this context, it is an exciting observation of soil research that the Indians of the Amazon basin used locally generated charcoal for the improvement of soil quality for hundreds of years (i.e. improving the water and ion binding of rich black soil) and that this carbon fraction was not easily decomposed.
Hydrothermal carbonisation
Besides charcoal formation, which is performed with high quality, dry biomass only, hydrothermal carbonization (HTC) is an especially promising process. The first experiments were carried out by Bergius, who, in 1913, had already described the hydrothermal transformation of cellulose into coal-like materials.9 More systematic investigations were performed by Berl and Schmidt in 1932, which varied the biomass source and treated the different samples, in the presence of water, at temperatures between 150 and 350 °C.10 The latter authors summarized, via a series of papers in 1932, the knowledge of those days about the emergence of coal. Later, Schuhmacher et al. analyzed the influence of pH on the outcome of the HTC reaction and found serious differences in the decomposition schemes, as identified by the C/H/O composition.
A renaissance in such experiments was started with reports on the low temperature hydrothermal synthesis of carbon spheres (highter than or equal to 200°C), and gave exciting nanostructures. It was also revealed that the presence of ternary components in complex biomass (such as orange peel or oak leaves) seriously alters decomposition schemes. Unexpectedly, an improvement in properties of the carbonaceous structures for certain applications was found, i.e. smaller structural size of carbon dispersions and porous networks, higher hydrophilicity of the surfaces, and higher capillarity.
For completeness, it must also be mentioned that, beyond coalification, the conversion of biomass under hydrothermal conditions is a widely examined process. These approaches aim for the recovery of liquid or gaseous fuel intermediates (like glucose, 5-hydroxymethylfurfural, methane, hydrogen etc.) from biomass, while the solid residues were, up until now, mostly treated as undesirable side products.
However, the described acceleration of HTC for coalification by a factor of 106–109 under rather soft conditions, down to a scale of hours, also makes it a considerable, technically-attractive alternative for the sequestration of carbon from biomass on large and ultra-large scales. Finally, to summarize the outcome of the optimization trials, catalyzed HTC required only the heating of a biomass dispersion under weakly acidic conditions in a closed reaction vessel for 4–24 h to temperatures of around 200 °C. This is indeed an extremely simple, cheap and easily scalable process. Besides that, HTC has a number of other practical advantages. HTC inherently requires wet starting products or biomass, as effective dehydration only occurs in the presence of water, plus the final carbon can be easily filtered from the reaction solution. This way, complicated drying schemes and costly isolation procedures can be conceptually avoided. In addition, under acidic conditions and below 200 °C, most of the original carbon stays bound to the final structure. Carbon structures produced by this route—either for deposit or materials use—are therefore the most CO2-efficient.
Carbon storage
Therefore, we strongly believe that the carbonization of fast growing plants is currently the most efficient process for removing atmospheric CO2, binding it into depositable carbon or even as useful solids.
For a negative atmospheric CO2 balance, the generated carbonaceous materials have to be deposited on a large scale, and potential carbon landfills may lay the foundations for chemical starting materials of the next century.
Another quite attractive application with immediate impact is their use as water- and ion-binding components to improve soil quality. This is a chemical process that is also found in nature, and carbonaceous soil is presumably the largest active carbon sink on earth. The proposed terra preta, i.e. artificial coal-enriched soil as a potential carbon sink of global dimensions, has already been mentioned in soil research, improving soil quality and plant growth at the same time. Instead of clearing the rainforest for questionable palm oil production, such a carbon-reinforced "turbo-rainforest" would produce at least 10 times the energy, but stored in carbon, whilst also being CO2-negative for the climate and supporting biodiversity at the same time.
Spending just 10% of our oil expenses on global CO2 sequestration would compensate for carbon fixation costs of US$44 per ton, a target which can, in the researchers' opinion, be quite easily met (HTC is essentially just heating an aqueous dispersion, where even the energy is generated by the process itself) and does not even consider the added value for the geosystem or agriculture.
Even in industrial countries such as Germany, only the treatment of highly defined waste biomass, such as from sugar-beet (4.3 Mt sugar per year), rapeseed production (3.5 Mt oil per year), or clarification sludge (3.0 Mt per year), has the potential to lower German CO2 output by about 10%. The low-tech processing of fast growing plants into non- or weakly-degradable peat-type carbon scaffolds by hydrothermal reaction cascades is therefore, in our opinion, a realistic artificial instrument for reducing atmospheric CO2.
New chemistry
More information:
Maria-Magdalena Titirici, Arne Thomas and Markus Antonietti, "Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem?", New J. Chem., 8th March 2007, DOI: 10.1039/b616045j
Article continues
posted by Biopact team at 3:30 PM 2 comments links to this post
