Scientists propose new geoengineering option: increasing ocean's alkalinity to soak up more carbon dioxide
 Researchers in Massachusetts and Pennsylvania are proposing a new method for reducing global warming that involves building a series of water desalination plants that remove hydrochloric acid (HCl) from the ocean and neutralize it through a reaction with silicate rocks. This removal would enhance the ability of the ocean to absorb carbon dioxide from the atmosphere. About 100 such plants - which essentially use the ocean as a giant carbon dioxide collector - could cause a 15 percent reduction in emissions over many years, they say. About 700 plants could offset all CO2 emissions. The technique could be deployed in case the dark scenario of 'abrupt climate change' were to be upon us.
Researchers in Massachusetts and Pennsylvania are proposing a new method for reducing global warming that involves building a series of water desalination plants that remove hydrochloric acid (HCl) from the ocean and neutralize it through a reaction with silicate rocks. This removal would enhance the ability of the ocean to absorb carbon dioxide from the atmosphere. About 100 such plants - which essentially use the ocean as a giant carbon dioxide collector - could cause a 15 percent reduction in emissions over many years, they say. About 700 plants could offset all CO2 emissions. The technique could be deployed in case the dark scenario of 'abrupt climate change' were to be upon us.The new idea is interesting, but competes with options that are far more cost-effective and energy efficient today, the researchers say, such as capturing carbon dioxide from large point sources (coal plants). A less costly and more efficient option still, is the production of carbon-negative biofuels and negative emissons via biomass with carbon-storage. However, given the threat of 'abrupt' and 'catastrophic' climate change, all possibilities must be looked at, even those that are not strictly cost-effective or efficient. The scientists' study is scheduled to appear in the Dec. 15 issue of ACS Environmental Science & Technology but is available as an ASAP open access article.
Scientists believe that excessive build-up of carbon dioxide in the air contributes to global warming. In addition to cutting down on carbon dioxide emissions by reducing the use of fossil fuels, researchers have focused on new technologies that remove the gas directly from the atmosphere.
Several of these geoengineering methods rely on natural processes that are emulated and strengthened in an artificial way. Some of these ideas are controversial and highly risky. An example would be the proposal to seed the oceans with iron, so that algae blooms are generated which sequester CO2. The idea has been rejected by scientists, environmentalists and international maritime organisations (previous post; for other risky proposals, see here and here). A safer geoengineering idea is to build 'artificial trees' which capture CO2 from the air by solvent regeneration cycles, to produce a pure stream of CO2 which can then be stored in geological formations. However, the process is very energy intensive (previous post).
Last but not least, a very natural, efficient and cost-effective geoengineering option consists of utilizing real trees to let them act as machines that clean up the atmosphere. The biomass stores CO2. If this biomass is then used for the production of fuels and energy, while the carbon is captured before, during or after the transformation, and thereafter stored underground, the fuels and energy become carbon-negative. These so-called 'bioenergy with carbon storage' (BECS) or negative emissions systems can be implemented safely and offer a cost-effective CO2 removal option because the energy obtained from these systems replaces fossil fuels while at the same time taking CO2 out of the atmosphere. Moreover, the energy required to capture and store the CO2 is generated by the system itself. Renewables like wind and solar, or nuclear power, are all 'carbon-neutral' at best because they do not add new emissions to the atmosphere. BECS systems go much further and actually take historic emissions out of it. Biopact readers are aware of the growing interest in these bio-based negative emissions energy concepts.
Despite the existence of several feasible options, researchers keep searching for alternative geoengineering methods to offset carbon dioxide, because, according to the latest IPCC synthesis, climate change is more serious than expected and is now said likely to result in 'abrupt' and 'irreversible' changes (previous post). To avert catastrophic climate change, all options must be considered, including less efficient techniques than can be deployed in a decentralised manner and thus contribute to a planetary effort.
Boosting ocean's CO2 uptake
In their new study, Kurt Zenz House and colleagues propose building hundreds of special water treatment facilities worldwide, in remote locations, that would remove hydrochloric acid from the ocean by electrolysis and neutralize the acid through reactions with silicate minerals or rocks (schematic, click to enlarge).
The reaction increases the alkalinity of the ocean and its ability to absorb carbon dioxide from the atmosphere. The process is similar to the natural weathering reactions that occur among silicate rocks but works at a much faster rate, the researchers say:
 sustainability :: ethanol :: biomass :: bioenergy :: biofuels :: greenhouse gas emissions :: carbon dioxide :: CCS :: BECS :: oceans :: geoengineering ::
sustainability :: ethanol :: biomass :: bioenergy :: biofuels :: greenhouse gas emissions :: carbon dioxide :: CCS :: BECS :: oceans :: geoengineering :: A range of efficiency scenarios indicates that the process should require 100–400 kJ of work per mol of CO2 captured and stored for relevant timescales. This means the process is energy intensive. The researchers suggest to utilize power from 'stranded energy sources' too remote to be useful for the direct needs of population centers.
But herein lies a problem. If these 'stranded energy sources' are fossil fuels, the energy required to desalinate the water may contribute to the release of more carbon dioxide than the method sequesters. The work input required for the overall process is expected to be between 1.5 and 3.5 times higher per unit of CO2 than the work required for postcombustion capture and geologic storage (CCS) of CO2 from a modern coal-fired power plant. BECS and carbon-negative bioenergy production is more cost-effective still, because it requires similar amounts of energy to capture and store CO2 compared to coal plants with CCS, while reducing atmospheric CO2 and resulting in net negative emissions (as opposed to merely reducing the amount of new emissions entering the atmosphere, as is the case in coal + CCS) (For a comparison of costs at different carbon prices, see: Christian Azar, Kristian Lindgren, Eric Larson and Kenneth Möllersten, "Carbon Capture and Storage From Fossil Fuels and Biomass – Costs and Potential Role in Stabilizing the Atmosphere", Climatic Change, Volume 74, Numbers 1-3 / January, 2006, DOI 10.1007/s10584-005-3484-7).
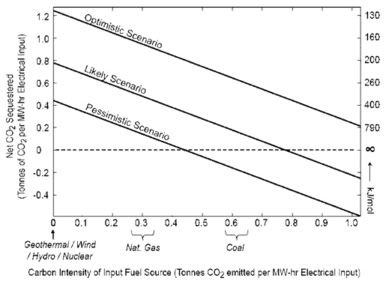
The new geoengineering technique will not be able to compete with either (coal + CC or biomass + CCS) because it is too energy intensive and risks relying on fossil fuels for its energy requirements. However, if low or zero-carbon renewables like wind, geothermal or hydropower were to be coupled to the system, it could become greener though (graph, click to enlarge).
Net CO2 sequestered (t) as a function of the carbon intensity of input fuel source for the process depicted using standard Icelandic basalt (BIR-1) to neutralize the HCl. The three lines correspond to different efficiency scenarios. The optimistic scenario assumes 10 molar NaCl solution with a 1-step electrochemical process at 60% efficiency for steps 1 and 2 and 15% productive heat recovery from step 3. The likely scenario assumes 10 molar NaCl solution, a 70% efficient electrolysis, a 70% efficient HCl fuel cell, and zero heat recovery from step 3. Finally, the pessimistic scenario assumes 0.6 molar NaCl, 50% efficiency for the electrolysis, 50% efficiency for the HCl fuel cell, and zero heat recovery. Also, this figure assumes the time scale of anthropogenic climate change is similar to the time scale for the surface ocean to remove excess alkalinity. The right-hand vertical axis is required work per net mole of CO2 sequestered.
However, an advantage of the method of electrolyzing seawater to enhance ocean CO2 uptake is that it can be performed in geographic regions with an abundance of zero and low carbon power sources. For example, stranded geothermal energy from active volcanic regions is a relatively inexpensive and carbon-free power source. Therefore, volcanic islands with large geothermal resources and large basalt deposits might be ideal locations. Wind turbines—whose general deployment is partially limited by intermittency of supply—are another interesting carbon-free power option because the process can be designed to operate only when an excess of wind power exists. Alternatively, the process could be powered by gas-turbines in oil-producing regions where natural gas is flared because the infrastructure required for long-distance transport is not available.
Research into actively capturing CO2 from the air by solvent regeneration cycles is ongoing (see the discussion of the 'artificial tree method'). The new method - enhancing ocean uptake of CO2 via seawater electrolysis - has some benefits over the solvent regeneration proposals. The goal of the solvent regeneration processes is to separate CO2(g) from air and produce a near pure stream of CO2(g) for compression to ~200 atm, transportation to a storage site, and injection into a geologic reservoir. In contrast, the CO2 in the process discussed by the scientists is chemically altered to a more stable state and permanently stored in the ocean. Hence it would not be necessary to locate and fully characterize a multitude of suitable geologic storage depositories for the captured CO2.
This is clearly an advantage over carbon sequestration from existing point sources such as coal plants. However, bio-energy with carbon storage systems can be decentralised, because biomass can be planted and planned at a selected site, close to geological storage depositories (unlike coal and fossil fuels, which are 'discovered' and remain where they are found, in a fixed place).
Technical and cost barriers
The electrolysis and HCl removal process process must overcome several technical hurdles before it can offset an appreciable quantity of CO2 emissions. The magnitude of the CO2 problem is daunting, and offsetting even 15% of global emissions by electrolysis of seawater would be a serious task. To offset 15% of annual carbon emissions (3.7 Gt CO2 or 1 Gt of carbon), 1014 moles of HCl would have to be removed from the ocean and neutralized per year. Seawater would have to be separated into acid and base at a global volumetric flow rate of ~6000 m3/s. Large sewage treatment facilities have a capacity of 60 m3/s. Thus, capturing and storing 3.7 Gt of CO2 annually by the process would require around 100 plants with a volumetric flow capacity similar to that of large sewage treatment facilities.
If the process were to be employed with artificial brine from mined halite deposits, then the volumetric flow rate requirements would be reduced by an order of magnitude. The chloralkali industry would have to grow for 50 years by 3.75% per year over and above the normal consumption growth from a base of 43 million t of Cl2 production in 2003. Chemical weathering would increase from 0.4 to 1.4 Gt of C per year over this same period. With 1020 moles of mineral NaCl in continental basins, there is a sufficient resource of mineral NaCl to offset many centuries of anthropogenic CO2 emissions using the standard Chloralkali process coupled with an HCl fuel cell and silicate rock dissolution.
Economical electrolysis of seawater is another technological challenge. The current cost of removing multivalent cations before running the Chloralkali process is high. Additionally, ohmic losses in seawater would need to be reduced, e.g., by concentrating through evaporation, boiling, or dissolution of mineral halite.
One potential undesirable consequence of employing this process directly with seawater would be the production of halogenated organics as a byproduct of the electrochemical reactions on seawater. During the electrolysis, some dissolved organic carbon (DOC) can be expected to be halogenated and some of this could be in the form of volatile stratospheric ozone-destroying compounds such as CH3Br3 and CH3Cl3. Even when the process employs artificial brine, there is evidence for generation of chlorinated organics from chloralkali plants due to leakage. Present estimates do not list the chloralkali process as a major contributor to the atmospheric chloroform flux.
Seawater electrolysis could significantly add to this contribution. The use of more concentrated NaCl solutions for electrolysis would reduce these emissions by limiting the availability of DOC. In any case, it will be important to quantify this unintended flux of bromine and chlorine to the atmosphere before any large scale implementation of this process proceeds, the researchers say.
Technologies currently exist that can offset or eliminate CO2 emissions from large point sources more cost-effectively than the process described here. In time, however, the most cost-effective CO2 mitigation schemes are likely to be fully utilized. If anthropogenic climate change is expected to remain a serious threat despite the fullest practical deployment of those schemes, then the process we discuss could provide the additional CO2 mitigation necessary to avoid further damage from climate change. If a technology based on this process is to be ready when and if needed, substantial laboratory and field research is needed to better understand the process’s effect on biogeochemical cycles and other unintended consequences; to develop efficient and robust large-scale hydrogen–chlorine fuel cells; and to develop processes to more efficiently separate seawater into acid and base with large throughput.Conclusion
Current estimates indicate that running the process is unlikely to be commercially viable in the near future. It is plausible, however, that the marginal cost for CO2 reduction will become high enough to make the process discussed here economically competitive. It is also worth considering the possibility that abrupt climate change will require a sudden and large-scale effort at CO2 mitigation. Under such a scenario, wide-scale deployment could be considered along with other geoengineering options to help avoid catastrophic climate change.
A variety of technologies will be used in this century to mitigate anthropogenic climate change. The process described by the scientists enhances the solubility of CO2 in the ocean by, in essence, electrochemically accelerating the natural chemical weathering reaction. The three key benefits of the process are the permanency guaranteed by the storage of CO2 in the ocean without acidification, the process’ capability to offset the CO2 emissions from any source including mobile point sources, and its capability to be performed in remote regions using stranded energy. Deployment of the process will be limited by any damage to local marine biota caused by local pH changes and rock dissolution products.
More efficient and cost-effective geoengineering options - such as BECS and negative emissions fuels and energy from biomass - will be implemented earlier than the new proposal. The bio-based methods were developed specifically in the context of the doom scenario of 'abrupt climate change'. However, if such a scenario really unfolds, even the more inefficient geoengineering ideas could find an opportunity for deployment.
References:
Kurt Zenz House,Christopher H. House, Daniel P. Schrag, and Michael J. Aziz, "Electrochemical Acceleration of Chemical Weathering as an Energetically Feasible Approach to Mitigating Anthropogenic Climate Change", Environ. Sci. Technol., ASAP Article, November 7, 2007, DOI: 10.1021/es0701816
Biopact: IPCC to warn of 'abrupt' climate change: emergency case for carbon-negative biofuels kicks in - November 16, 2007
Biopact: Scientists propose artificial trees to scrub CO2 out of the atmosphere - but the real thing could be smarter - October 04, 2007
Biopact: International maritime body rejects risky ocean geoengineering - November 09, 2007
Biopact: Simulation shows geoengineering is very risky - June 05, 2007
 --------------
--------------
 The Royal Society of Chemistry has announced it will launch a new journal in summer 2008, Energy & Environmental Science, which will distinctly address both energy and environmental issues. In recognition of the importance of research in this subject, and the need for knowledge transfer between scientists throughout the world, from launch the RSC will make issues of Energy & Environmental Science available free of charge to readers via its
The Royal Society of Chemistry has announced it will launch a new journal in summer 2008, Energy & Environmental Science, which will distinctly address both energy and environmental issues. In recognition of the importance of research in this subject, and the need for knowledge transfer between scientists throughout the world, from launch the RSC will make issues of Energy & Environmental Science available free of charge to readers via its 







3 Comments:
I take it that such a process is only seen as a fall back... as the rest of the world are looking at reducing carbon output...
Yep, the researchers say it's an option that would only be feasible if we face catastrophic climate change and need to act quickly and with everything we've got.
But mind you, the IPCC recently said we may in fact already be on the brink of this dreadful and feared 'abrupt climate change' (ACC) scenario.
and what would the total alk increase be to the ocean? a .5dkh increase and corals will not respond nicely. for the most part they have not experience any fluctuation in alkalinity.
it would also slightly change the CNP ratio, no?
eric
www.glassboxlife.blogspot.com
Post a Comment
Links to this post:
Create a Link
<< Home