European Science Foundation launches microbial biofilms project - applications in bioenergy
Yet biofilms also have equal potential for good behaviour, in particular as agents of self-purification in streams and rivers, waste and pollution treatment, or generation of carbon-neutral electricity. These critical properties are derived from the existence of the protective slimy matrix within which members of the community live, preventing attack from both the immune system and antibiotics, but at the same time shielding them from toxic contaminants while breaking down waste or effluent.
The study of biofilms has emerged over the last three decades in various disciplines such as biotechnology, bioengineering or infectious disease research, leading to rapid progress, but also fragmentation and duplication of effort. The European Science Foundation (ESF) has stepped in to unite Europe's effort and bring together scientists with the required skills in relevant fields such as genetics, molecular biology, microscopy, medical microbiology, environmental science and ecology.
The programme began with an Exploratory Workshop in September 2007, titled 'Valuing Biofilm Services: the Beauty and the Beast', leading to a proposal for a new body to coordinate activities, the European Biofilm Net (EBN). The ESF workshop highlighted the huge potential and importance of biofilms, and also drew attention to exciting work unravelling the complex genetic and cellular interactions within these small yet teeming communities.
The ESF workshop highlighted greater understanding of the complex interactions within biofilms, which often comprise not just one species of bacteria, but a whole host of different micro-organisms, including archaea, protozoa, fungi, and even tiny metazoa actually comprising multiple cells. Many biofilms are in fact complete micro-ecosystems, within which there is competition as well as cooperation, and unraveling the interactions will reveal valuable insights into how these evolved.
 The Workshop's convenor Tom J. Battin, from the University of Vienna, pointed out that there was great excitement about an emerging application that could have potential for green energy production - the use of biofilms to power microbial fuel cells (MFCs, introduction) whose fuel could be wastewater or any type of waste biomass, as outlined at the ESF workshop by Cristian Picioreanu, Delft University of Technology. This exploits the ability of bacteria to transfer electrons to metals, which can be the cathode of a fuel cell, via the minute tentacles called phili extending from their surface.
The Workshop's convenor Tom J. Battin, from the University of Vienna, pointed out that there was great excitement about an emerging application that could have potential for green energy production - the use of biofilms to power microbial fuel cells (MFCs, introduction) whose fuel could be wastewater or any type of waste biomass, as outlined at the ESF workshop by Cristian Picioreanu, Delft University of Technology. This exploits the ability of bacteria to transfer electrons to metals, which can be the cathode of a fuel cell, via the minute tentacles called phili extending from their surface.Microbiologists recently found that the power output of microbial fuel cells can be boosted more than 10-fold by letting the bacteria congregate into such slimy biofilms (schematic shows set-up of a biofilm based MFC, click to enlarge):
 energy :: sustainability :: biomass :: bioenergy :: biofuels :: water treatment :: microbial fuel cell :: biofilm ::microbiology ::
energy :: sustainability :: biomass :: bioenergy :: biofuels :: water treatment :: microbial fuel cell :: biofilm ::microbiology :: A typical fuel cell converts fuels to electricity without the need for combustion and microbial fuel cells work the same way. They usually comprise two compartments, or cells, which are separated by an electrically insulating membrane. In one compartment, microorganisms pull electrons and protons from some sort of fuel—such as waste organic matter. These protons and electrons are attracted to molecules in the second compartment—usually oxygen—and will move towards those molecules. The protons do this by passing through the membrane. But the electrons can’t go through the membrane and so must travel via an alternate route—a wire, or electrode that connects the two compartments. It is this flow of electrons through the electrode that supplies power.
Microbial fuels cells harness the electron shuttling that occurs in the energy-making pathway of certain bacteria. In the energy-making pathway of most animals, electrons and protons are also shuttled about, and usually electrons are passed to oxygen brought in through the lungs. Early microbial fuel cells intercepted the bacteria’s electron shuttling with compounds called 'mediators,' which would penetrate the bacteria, snatch electrons and then transfer them to the metal electrode. But the compounds typically used as mediators are often expensive and toxic. A more recent and efficient approach has been to use microbes that can pass electrons directly to a metal electrode.
These “metal-reducing” bacteria are ideal for fuel cells, especially species of Geobacter and Rhodoferax, microbes that evolved means to transfer electrons to metals in the surrounding environment. The microbes use thin wire-like growths, several cell lengths long, that extend from their cell membrane out into the environment. Many bacteria have these extended structures—called pili—they usually use the hair-like extensions to attach to other cells or surfaces. But Geobacter uses pili to transfer electrons onto iron in the surrounding soil. These so-called 'microbial nanowires' also seem to be critical for Geobacter to form a biofilm.
While investigating the microbes’ electron transfer mechanism, microbiologists recently created mutant Geobacter that don’t have the gene for making the phili, yet the microbes still produced electricity when placed in a fuel cell. The researchers suspected that a membrane protein that was part of the microbe’s energy-making pathway was also able to transfer electrons directly to the metal electrode.
The microbes were lined up in a single, thin layer along the electrode and it seemed that either the nanowires or the membrane protein had to be in direct contact with the electrode for electron transfer to occur.
But then the researchers tweaked the fuel cell so the second compartment could take as many electrons as the microbes could provide. To their surprise, the power output increased dramatically and Geobacter began to grow on the electrode in a thick, sticky mass known as a biofilm. However, the mutant Geobacter that couldn’t make phili couldn’t congregate into a biofilm. The mutants produced electricity at a much slower rate and so it seemed that Geobacter’s phili are essential for making a biofilm.
Many bacteria form biofilms — the gluey matrix of sugars serves to anchor free - floating microbes to various surfaces, such as teeth, a refrigerator drawer or rocks in a stream. Biofilms are usually the bane of those who encounter them — they cause tooth decay, ruin the hulls of ships and can cause serious health problems when they glom onto medical implant devices such as catheters. But in this instance, the biofilm is a good thing. It seems to act as one big, slimy, conductive mat, allowing electrons to be transferred by bacteria that aren’t in direct contact with the electrode.
Further experiments have confirmed that microbes in the center of the biofilm — too far from the electrode to reach it themselves — transfer electrons at the same rate as microbes that are closer to the edge. It is a finding the scientists would have predicted.
It makes sense that Geobacter is in direct contact with the electrode to pass electrons. But now there are these big slime layers — big red glops of Geobacter growing on the electrode — and they are all passing electrons. How the electrons are transferred through the gooey matrix isn’t clear, but this is what is being investigated currently. It does seem clear that improved methods for generating electricity via microbial fuel cells may be only a few slimy steps away.
The ESF Biofilm workshop delved into biofilm's role chronic infections, including killers such as cystic fibrosis, and endocarditis in the heart. Batin explains that in cystic fibrosis, excess mucus production in the airways gives sanctuary to bacteria such as Pseudomonas aeruginosa, which actually mop up the dead carcasses of white blood cells sent by the immune system, enabling them to construct their protective biofilm coat. In this case the immune system is the architect of its own problems, helping create the shield used to repel its own agents, as well as resisting antibiotics. Indeed resistance against antibiotics is itself one of the biggest problems of all associated with biofilms, Battin noted.
This becomes particularly dramatic for endocarditis patients, as was outlined at the workshop by Annette Moter from the Charite in Berlin, said Battin. Endocarditis is a rare but serious disease in which one of the four heart valves, the heart lining, or heart muscle, are infected by a bacterial biofilm, often comprising streptococci, and become inflamed. As the biofilms are resistant to antibiotics and the immune system�s white blood cells, very often the only remedy is surgery, to replace a damaged valve, which can itself cause problems. The hope is that greater understanding will yield new drugs that reach the infected heart valve and break up the biofilm.
As Battin pointed out, biofilms can pose a big problem in large-scale water treatment plants, and yet for the very same reasons can play a positive role in the very same process, breaking down contaminants in waste and natural waters, for example. Further research will help ensure that the positive role is accentuated, while avoiding the problems.
The ESF Workshop, 'Valuing Biofilm Services: the Beauty and the Beast', was held 19-22 September at the interuniversity research center WasserCluster Lunz, Austria. This is being followed by a proposal for the EBN, with the specific objectives of developing both laboratory and computational techniques, while integrating relevant fields such as system biology or ecology and evolution, to create the interdisciplinary platform for a new era of biofilm research.
Each year, ESF supports approximately 50 Exploratory Workshops across all scientific domains. These small, interactive group sessions are aimed at opening up new directions in research to explore new fields with a potential impact on developments in science.
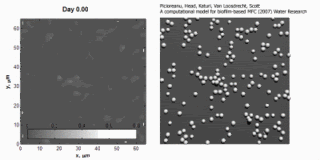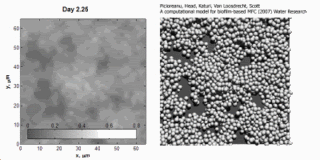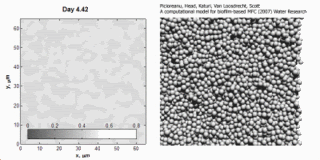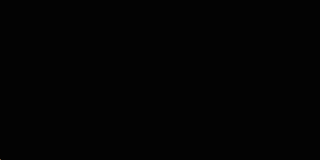
Image and animation: animation shows simulation of biofilm development over a 7-day period. Courtesy: Cristian Picioreanu, TU Delft.
References:
European Science Foundation Exploratory Workshop: Valuing Biofilm Services: the Beauty and the Beast.
Eurekalert: Microbial biofilms evoke Jekyll & Hyde effects - 26-Oct-2007
Cristian Picioreanu et al., "A computational model for biofilm-based microbial fuel cells", Water Research, 41(13):2921-2940, 29 May 2007
Technical University of Delft: Biofilm modeling research group.
Biopact: Microbial fuel cell development speeds up: from biopower in space to the developing world - September 30, 2007
 --------------
--------------
 Biopact is moving to a new server this weekend, so at times the site may be difficult to access or temporarily offline. We should be up and running again on Monday.
Biopact is moving to a new server this weekend, so at times the site may be difficult to access or temporarily offline. We should be up and running again on Monday.








0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home