
 Malaysian company Ecofuture Bhd makes renewable products from palm oil residues such as empty fruit bunches and fibers (more here). It expects the revenue contribution of these products to grow by 10% this year, due to growing overseas demand, says executive chairman Jang Lim Kuang. 95% of the group's export earnings come from these products which include natural oil palm fibre strands and biodegradable mulching and soil erosion geotextile mats.
Bernama - June 20, 2007. Malaysian company Ecofuture Bhd makes renewable products from palm oil residues such as empty fruit bunches and fibers (more here). It expects the revenue contribution of these products to grow by 10% this year, due to growing overseas demand, says executive chairman Jang Lim Kuang. 95% of the group's export earnings come from these products which include natural oil palm fibre strands and biodegradable mulching and soil erosion geotextile mats.
Bernama - June 20, 2007.
 Argent Energy, a British producer of waste-oil based biodiesel, announced its intention to seek a listing on London's AIM via a placing of new and existing ordinary shares with institutional investors. Argent plans to use the proceeds to construct the first phase of its proposed 150,000 tonnes (170 million litres) plant at Ellesmere Port, near Chester, and to develop further plans for a 75,000 tonnes (85 million litres) plant in New Zealand.
Argent Energy - June 20, 2007. Argent Energy, a British producer of waste-oil based biodiesel, announced its intention to seek a listing on London's AIM via a placing of new and existing ordinary shares with institutional investors. Argent plans to use the proceeds to construct the first phase of its proposed 150,000 tonnes (170 million litres) plant at Ellesmere Port, near Chester, and to develop further plans for a 75,000 tonnes (85 million litres) plant in New Zealand.
Argent Energy - June 20, 2007.
 The first conference of the European Biomass Co-firing Network will be held in Budapest, Hungary, from 2 to 4 July 2007. The purpose of the conference is to bring together scientists, engineers and members of public institutions to present the current state-of-the-art on biomass co-firing. Participants will also discuss future trends and directions in order to promote awareness of this technology as a sustainable energy supply, which could decrease the dependency on fossil fuels and guarantee a decentralised source of energy in Europe. The conference is supported by the EU-funded NETBIOCOF (Integrated European Network for Biomass Co-firing) project.
NetBioCof - June 19, 2007. The first conference of the European Biomass Co-firing Network will be held in Budapest, Hungary, from 2 to 4 July 2007. The purpose of the conference is to bring together scientists, engineers and members of public institutions to present the current state-of-the-art on biomass co-firing. Participants will also discuss future trends and directions in order to promote awareness of this technology as a sustainable energy supply, which could decrease the dependency on fossil fuels and guarantee a decentralised source of energy in Europe. The conference is supported by the EU-funded NETBIOCOF (Integrated European Network for Biomass Co-firing) project.
NetBioCof - June 19, 2007.
 Green Energy Resources predicts US$50 per ton biomass woodchip prices within the next twelve months. The current US price level is between $25-32 per ton. Demand caused by the 25-30 new power plants planned in New England by 2010 does not include industry, institutions, universities, hospitals or conversions from natural gas, or cellulostic ethanol. Procurement of woodchips will be based on the delivery capacity of suppliers not local prices for the first time in history. Green Energy has been positioning in New England with rail and port locations to meet the anticipated sector expansion.
MarketWire - June 19, 2007. Green Energy Resources predicts US$50 per ton biomass woodchip prices within the next twelve months. The current US price level is between $25-32 per ton. Demand caused by the 25-30 new power plants planned in New England by 2010 does not include industry, institutions, universities, hospitals or conversions from natural gas, or cellulostic ethanol. Procurement of woodchips will be based on the delivery capacity of suppliers not local prices for the first time in history. Green Energy has been positioning in New England with rail and port locations to meet the anticipated sector expansion.
MarketWire - June 19, 2007.
 In the first major initiative in the US to build a grassroots communications network for the advancement of biofuels adoption, a new national association called The American Biofuels Council (ABC) has been formed.
American Biofuels Council - June 19, 2007. In the first major initiative in the US to build a grassroots communications network for the advancement of biofuels adoption, a new national association called The American Biofuels Council (ABC) has been formed.
American Biofuels Council - June 19, 2007.
 The Novi Sad-based Jerković Group, in partnership with the Austrian Christof Group, are to invest about €48 million (US$64.3m) in a biodiesel plant in Serbia.
Property Xpress - June 19, 2007. The Novi Sad-based Jerković Group, in partnership with the Austrian Christof Group, are to invest about €48 million (US$64.3m) in a biodiesel plant in Serbia.
Property Xpress - June 19, 2007.
 Biodiesel producer D1 Oils, known for its vast jatropha plantations in Africa and Asia, is to invest CNY 500 to 700 million (€48.9-68.4 / US$65.5-91.7) to build a refinery in Guangxi Zhuang autonomous region, in what is expected to be the first biodiesel plant in the country using jatropha oil as a feedstock.
South China Morning Post - June 18, 2007. Biodiesel producer D1 Oils, known for its vast jatropha plantations in Africa and Asia, is to invest CNY 500 to 700 million (€48.9-68.4 / US$65.5-91.7) to build a refinery in Guangxi Zhuang autonomous region, in what is expected to be the first biodiesel plant in the country using jatropha oil as a feedstock.
South China Morning Post - June 18, 2007.
 After Brazil announced a record sugar crop for this year, with a decline in both ethanol and sugar prices as a result, India too is now preparing for a bumper harvest, a senior economist with the International Sugar Organization said. Raw sugar prices could fall further towards 8 cents per lb in coming months, after their 30% drop so far this year. Converting the global surplus, estimated to be 4 million tonnes, into ethanol may offer a way out of the downward trend.
Economic Times India - June 18, 2007. After Brazil announced a record sugar crop for this year, with a decline in both ethanol and sugar prices as a result, India too is now preparing for a bumper harvest, a senior economist with the International Sugar Organization said. Raw sugar prices could fall further towards 8 cents per lb in coming months, after their 30% drop so far this year. Converting the global surplus, estimated to be 4 million tonnes, into ethanol may offer a way out of the downward trend.
Economic Times India - June 18, 2007.
 After Brazil announced a record sugar crop for this year, with a decline in both ethanol and sugar prices as a result, India too is now preparing for a bumper harvest, a senior economist with the International Sugar Organization said. Raw sugar prices could fall further towards 8 cents per lb in coming months, after their 30% drop so far this year. Converting the global surplus, estimated to be 4 million tonnes, into ethanol may offer a way out of the downward trend.
Economic Times India - June 18, 2007. After Brazil announced a record sugar crop for this year, with a decline in both ethanol and sugar prices as a result, India too is now preparing for a bumper harvest, a senior economist with the International Sugar Organization said. Raw sugar prices could fall further towards 8 cents per lb in coming months, after their 30% drop so far this year. Converting the global surplus, estimated to be 4 million tonnes, into ethanol may offer a way out of the downward trend.
Economic Times India - June 18, 2007.
 A report from the US Department of Agriculture Foreign Agricultural Services (USDA FAS) estimates that the production of ethanol in China will reach 1.45 million tonnes (484 million gallons US) in 2007, up 12% from 1.3 million tonnes in 2006. Plans are to increase ethanol feedstocks from non-arable lands making the use of tuber crops such as cassava and sweet sorghum.
USDA-FAS - June 17, 2007. A report from the US Department of Agriculture Foreign Agricultural Services (USDA FAS) estimates that the production of ethanol in China will reach 1.45 million tonnes (484 million gallons US) in 2007, up 12% from 1.3 million tonnes in 2006. Plans are to increase ethanol feedstocks from non-arable lands making the use of tuber crops such as cassava and sweet sorghum.
USDA-FAS - June 17, 2007.
 The Iowa State University's Extension Bioeconomy Task Force carried out a round of discussions on the bioeconomy with citizens of the state. Results indicate most people see a bright future for the new economy, others are cautious and take on a distanced, more objective view. The potential for jobs and economic development were the most important opportunities identified by the panels. Iowa is the leading producer of corn based ethanol in the US.
Iowa State University - June 16, 2007. The Iowa State University's Extension Bioeconomy Task Force carried out a round of discussions on the bioeconomy with citizens of the state. Results indicate most people see a bright future for the new economy, others are cautious and take on a distanced, more objective view. The potential for jobs and economic development were the most important opportunities identified by the panels. Iowa is the leading producer of corn based ethanol in the US.
Iowa State University - June 16, 2007.
 Biofuel producer D1 Oils Plc, known for establishing large jatropha plantations on (degraded land) in Africa and Asia, said it was in advanced talks with an unnamed party regarding a strategic collaboration, sending its shares up 7 percent, after press reports linking it with BP. Firms like BP and other large petroleum companies are keen to secure a supply of biofuel to meet UK government regulations that 5 percent of automotive fuel must be made up of biofuels by 2010.
Reuters UK - June 15, 2007. Biofuel producer D1 Oils Plc, known for establishing large jatropha plantations on (degraded land) in Africa and Asia, said it was in advanced talks with an unnamed party regarding a strategic collaboration, sending its shares up 7 percent, after press reports linking it with BP. Firms like BP and other large petroleum companies are keen to secure a supply of biofuel to meet UK government regulations that 5 percent of automotive fuel must be made up of biofuels by 2010.
Reuters UK - June 15, 2007.
 Jean Ziegler, a U.N. special rapporteur on the right to food, told a news briefing held on the sidelines of the U.N. Human Rights Council that "there is a great danger for the right to food by the development of biofuels". His comments contradict a report published earlier by a consortium of UN agencies, which said biofuels could boost the food security of the poor.
Reuters - June 15, 2007. Jean Ziegler, a U.N. special rapporteur on the right to food, told a news briefing held on the sidelines of the U.N. Human Rights Council that "there is a great danger for the right to food by the development of biofuels". His comments contradict a report published earlier by a consortium of UN agencies, which said biofuels could boost the food security of the poor.
Reuters - June 15, 2007.
 The county of Chicheng in China's Hebei Province recently signed a cooperative contract with the Australian investment and advisory firm Babcock & Brown to invest RMB480 million (€47.2/US$62.9 million) in a biomass power project, state media reported today.
Interfax China - June 14, 2007. The county of Chicheng in China's Hebei Province recently signed a cooperative contract with the Australian investment and advisory firm Babcock & Brown to invest RMB480 million (€47.2/US$62.9 million) in a biomass power project, state media reported today.
Interfax China - June 14, 2007.
 A new two-stroke ICE engine developed by NEVIS Engine Company Ltd. may nearly double fuel efficiency and lower emissions. Moreover, the engine's versatile design means it can be configured to be fuelled not only by gasoline but also by diesel, hydrogen and biofuels.
PRWeb - June 14, 2007. A new two-stroke ICE engine developed by NEVIS Engine Company Ltd. may nearly double fuel efficiency and lower emissions. Moreover, the engine's versatile design means it can be configured to be fuelled not only by gasoline but also by diesel, hydrogen and biofuels.
PRWeb - June 14, 2007.
 Houston-based Gulf Ethanol Corp., announced it will develop sorghum as an alternative feedstock for the production of cellulosic ethanol. Scientists have developed drought tolerant, high-yield varieties of the crop that would grow well in the drier parts of the U.S. and reduce reliance on corn.
Business Wire - June 14, 2007. Houston-based Gulf Ethanol Corp., announced it will develop sorghum as an alternative feedstock for the production of cellulosic ethanol. Scientists have developed drought tolerant, high-yield varieties of the crop that would grow well in the drier parts of the U.S. and reduce reliance on corn.
Business Wire - June 14, 2007.
 Bulgaria's Rompetrol Rafinare is to start delivering Euro 4 grade diesel fuel with a 2% biodiesel content to its domestic market starting June 25, 2007. The same company recently started to distributing Super Ethanol E85 from its own brand and Dyneff brand filling stations in France. It is building a 2500 ton/month, €13.5/US$18 million biodiesel facility at its Petromidia refinery.
BBJ - June 13, 2007. Bulgaria's Rompetrol Rafinare is to start delivering Euro 4 grade diesel fuel with a 2% biodiesel content to its domestic market starting June 25, 2007. The same company recently started to distributing Super Ethanol E85 from its own brand and Dyneff brand filling stations in France. It is building a 2500 ton/month, €13.5/US$18 million biodiesel facility at its Petromidia refinery.
BBJ - June 13, 2007.
 San Diego Gas & Electric (SDG&E), a utility serving 3.4 million customers, announced it has signed a supply contract with Envirepel Energy, Inc. for renewable biomass energy that will be online by October 2007. Bioenergy is part of a 300MW fraction of SDG&E's portfolio of renewable resources.
San Diego Gas & Electric - June 13, 2007. San Diego Gas & Electric (SDG&E), a utility serving 3.4 million customers, announced it has signed a supply contract with Envirepel Energy, Inc. for renewable biomass energy that will be online by October 2007. Bioenergy is part of a 300MW fraction of SDG&E's portfolio of renewable resources.
San Diego Gas & Electric - June 13, 2007.
 Cycleenergy, an Austrian bioenergy group, closed €6.7 million in equity financing for expansion of its biomass and biogas power plant activities in Central and Eastern Europe. The company is currently completing construction of a 5.5 MW (nominal) woodchip fired biomass facility in northern Austria and has a total of over 150 MW of biomass and biogas combined heat and power (CHP) projects across Central Europe in the pipeline.
Cycleenergy Biopower [*.pdf] - June 12, 2007. Cycleenergy, an Austrian bioenergy group, closed €6.7 million in equity financing for expansion of its biomass and biogas power plant activities in Central and Eastern Europe. The company is currently completing construction of a 5.5 MW (nominal) woodchip fired biomass facility in northern Austria and has a total of over 150 MW of biomass and biogas combined heat and power (CHP) projects across Central Europe in the pipeline.
Cycleenergy Biopower [*.pdf] - June 12, 2007.
 The government of Taiwan unveils its plan to promote green energy, with all government vehicles in Taipei switching to E3 ethanol gasoline by September and biofuel expected to be available at all gas stations nationwide by 2011.
Taipei Times - June 12, 2007. The government of Taiwan unveils its plan to promote green energy, with all government vehicles in Taipei switching to E3 ethanol gasoline by September and biofuel expected to be available at all gas stations nationwide by 2011.
Taipei Times - June 12, 2007.
 A large-scale biogas production project is on scheme in Vienna. 17,000 tonnes of organic municipal waste will be converted into biogas that will save up to 3000 tonnes of CO2. 1.7 million cubic meters of biogas will be generated that will be converted into 11.200 MWh of electricity per year in a CHP plant, the heat of which will be used by 600 Viennese households. The €13 million project will come online later this year.
Wien Magazine [*German] - June 11, 2007. A large-scale biogas production project is on scheme in Vienna. 17,000 tonnes of organic municipal waste will be converted into biogas that will save up to 3000 tonnes of CO2. 1.7 million cubic meters of biogas will be generated that will be converted into 11.200 MWh of electricity per year in a CHP plant, the heat of which will be used by 600 Viennese households. The €13 million project will come online later this year.
Wien Magazine [*German] - June 11, 2007.
 The annual biodiesel market in Bulgaria may grow to 400 000 tons in two to three years, a report by the Oxford Business Group says. The figure would represent a 300-per cent increase compared to 2006 when 140 000 tons of biodiesel were produced in Bulgaria. This also means that biofuel usage in Bulgaria will account for 5.75 per cent of all fuel consumption by 2010, as required by the European Commission. A total of 25 biofuel producing plants operate in Bulgaria at present.
Sofia Echo - June 11, 2007. The annual biodiesel market in Bulgaria may grow to 400 000 tons in two to three years, a report by the Oxford Business Group says. The figure would represent a 300-per cent increase compared to 2006 when 140 000 tons of biodiesel were produced in Bulgaria. This also means that biofuel usage in Bulgaria will account for 5.75 per cent of all fuel consumption by 2010, as required by the European Commission. A total of 25 biofuel producing plants operate in Bulgaria at present.
Sofia Echo - June 11, 2007.
 The Jordan Biogas Company in Ruseifa is currently conducting negotiations with the government of Finland to sell CER's under the UN's Clean Development Mechanism obtained from biogas generated at the Ruseifa landfill.
Mena FN - June 11, 2007. The Jordan Biogas Company in Ruseifa is currently conducting negotiations with the government of Finland to sell CER's under the UN's Clean Development Mechanism obtained from biogas generated at the Ruseifa landfill.
Mena FN - June 11, 2007.
 Major European bank BNP Paribas will launch an investment company called Agrinvest this month to tap into the increased global demand for biofuels and rising consumption in Asia and emerging Europe.
CityWire - June 8, 2007. Major European bank BNP Paribas will launch an investment company called Agrinvest this month to tap into the increased global demand for biofuels and rising consumption in Asia and emerging Europe.
CityWire - June 8, 2007.
 Malaysian particleboard maker HeveaBoard Bhd expects to save some 12 million ringgit (€2.6/US$3.4 million) a year on fuel as its second plant is set to utilise biomass energy instead of fossil fuel. This would help improve operating margins, group managing director Tenson Yoong Tein Seng said. HeveaBoard, which commissioned the second plant last October, expects capacity utilisation to reach 70% by end of this year.
The Star - June 8, 2007. Malaysian particleboard maker HeveaBoard Bhd expects to save some 12 million ringgit (€2.6/US$3.4 million) a year on fuel as its second plant is set to utilise biomass energy instead of fossil fuel. This would help improve operating margins, group managing director Tenson Yoong Tein Seng said. HeveaBoard, which commissioned the second plant last October, expects capacity utilisation to reach 70% by end of this year.
The Star - June 8, 2007.
 Japan's Itochu Corp will team up with Brazilian state-run oil firm Petroleo Brasileiro SA to produce sugar cane-based bioethanol for biofuels, with plans to start exporting the biofuel to Japan around 2010. Itochu and Petrobras will grow sugarcane as well as build five to seven refineries in the northeastern state of Pernambuco. The two aim to produce 270 million liters (71.3 million gallons) of bioethanol a year, and target sales of around 130 billion yen (€800million / US$1billion) from exports of the products to Japan.
Forbes - June 8, 2007. Japan's Itochu Corp will team up with Brazilian state-run oil firm Petroleo Brasileiro SA to produce sugar cane-based bioethanol for biofuels, with plans to start exporting the biofuel to Japan around 2010. Itochu and Petrobras will grow sugarcane as well as build five to seven refineries in the northeastern state of Pernambuco. The two aim to produce 270 million liters (71.3 million gallons) of bioethanol a year, and target sales of around 130 billion yen (€800million / US$1billion) from exports of the products to Japan.
Forbes - June 8, 2007.
 Italian refining group Saras is building one of Spain's largest flexible biodiesel plants. The 200,000 ton per year factory in Cartagena can handle a variety of vegetable oils. The plant is due to start up in 2008 and will rely on European as well as imported feedstocks such as palm oil.
Reuters - June 7, 2007. Italian refining group Saras is building one of Spain's largest flexible biodiesel plants. The 200,000 ton per year factory in Cartagena can handle a variety of vegetable oils. The plant is due to start up in 2008 and will rely on European as well as imported feedstocks such as palm oil.
Reuters - June 7, 2007.
 The University of New Hampshire's Biodiesel Group is to test a fully automated process to convert waste vegetable oil into biodiesel. It has partnered with MPB Bioenergy, whose small-scale processor will be used in the trials.
UNH Biodiesel Group - June 7, 2007. The University of New Hampshire's Biodiesel Group is to test a fully automated process to convert waste vegetable oil into biodiesel. It has partnered with MPB Bioenergy, whose small-scale processor will be used in the trials.
UNH Biodiesel Group - June 7, 2007.
 According to the Barbados Agricultural Management Company (BAMC), the Caribbean island state has a large enough potential to meet both its domestic ethanol needs (E10) and to export to international markets. BAMC is working with state actors to develop an entirely green biofuel production process based on bagasse and biomass.
The Barbados Advocate - June 6, 2007. According to the Barbados Agricultural Management Company (BAMC), the Caribbean island state has a large enough potential to meet both its domestic ethanol needs (E10) and to export to international markets. BAMC is working with state actors to develop an entirely green biofuel production process based on bagasse and biomass.
The Barbados Advocate - June 6, 2007.

|
|
|
|
|
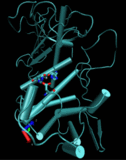 University at Buffalo chemists report the discovery of a central mechanism responsible for the action of the powerful biological catalysts known as enzymes. They published their results in an open access article in the journal Biochemistry. The findings surprised many enzymologists.
University at Buffalo chemists report the discovery of a central mechanism responsible for the action of the powerful biological catalysts known as enzymes. They published their results in an open access article in the journal Biochemistry. The findings surprised many enzymologists. bioenergy :: biofuels :: energy :: sustainability :: biomass :: bioconversion :: biorefining :: enzyme :: catalysis ::
bioenergy :: biofuels :: energy :: sustainability :: biomass :: bioconversion :: biorefining :: enzyme :: catalysis ::  --------------
--------------
 Malaysian company Ecofuture Bhd makes renewable products from palm oil residues such as empty fruit bunches and fibers (
Malaysian company Ecofuture Bhd makes renewable products from palm oil residues such as empty fruit bunches and fibers (







0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home