Genome sequencing reveals how bacterium selects enzymes for cellulase, key to viable ethanol production
 As the push towards alternative energy sources heats up, researchers at the University of Rochester have for the first time identified how genes responsible for biomass breakdown are turned on in a microorganism that produces valuable ethanol from biomass sources such as grass and corn stalks.
As the push towards alternative energy sources heats up, researchers at the University of Rochester have for the first time identified how genes responsible for biomass breakdown are turned on in a microorganism that produces valuable ethanol from biomass sources such as grass and corn stalks.Cellulose-rich waste products from agriculture and forestry, such as grass clippings and wood chips — once thought too difficult to turn into ethanol — may soon be fodder for hungry, gene-tweaked bacteria who convert the biomass into useful bio-products and fuels.
The findings in today's Proceedings of the National Academy of Sciences may empower scientists to engineer ethanol-producing 'super-organisms' that can make clean-burning fuel from the billions of tons of biomass that are produced each year and that remain unused.
"This is the first revelation of how a bacterium chooses from its more than 100 enzymes to break down a particular biomass. Once we know how a bacterium targets a particular type of biomass, we should be able to boost that process to draw ethanol from biomass far more efficiently that we can today." - David H. Wu, professor in the Department of Chemical Engineering at the University of Rochester.Ethanol holds the promise of a clean, renewable alternative to fossil fuels, but deriving it from ligno-cellulose is difficult. Producing it from corn is the easiest method, but doing so on a large scale would drive up the price of corn, corn starch, and even tangential foods like beef, since cows are fed on corn—not to mention all the energy spent fertilizing, maintaining, and harvesting a crop like corn. Conversely, deriving ethanol from plant materials such as the corn stalks and wood chips is challenging because the plants' cellulose is a very tough substance to break down, making for an inefficient process:
 bioenergy :: biofuels :: energy :: sustainability :: biomass :: bacteria :: cellulase :: cellulose :: ethanol :: biochemistry :: genome ::
bioenergy :: biofuels :: energy :: sustainability :: biomass :: bacteria :: cellulase :: cellulose :: ethanol :: biochemistry :: genome :: Wu's technique may prove much more effective than traditional methods. Instead of using separate steps to break down biomass into glucose and ferment the glucose into ethanol, as is currently done, Wu is working on a way to make a bacterium break down and ferment plant biomass efficiently in just one step.
Wu investigated C. thermocellum, which is a microorganism that has that ability to turn biomass into ethanol in one step, but is not used at the industrial scale yet because the first step, breaking down the plant's cellulose, is much too inefficient. The key, Wu surmised, is to find out what enzymes the bacterium uses to accomplish its feat, and then boost its ability to produce those enzymes. The problem, however, lies in the fact that C. thermocellum uses more than 100 enzymes, and any of the millions of combinations of them may be the magic mixture to break down a particular biomass.
So, Wu decided to make the bacterium do the work for him.
"The bacteria know how to express just the right genes to break down any particular biomass substrate, and we wanted to know how they know to turn on and off just the right genes at the right time to do the trick," says Wu. "We found the bacterium essentially throws the whole bowl of spaghetti at the wall, sees what sticks, and then makes a lot of that particular noodle."
C. thermocelllum produces low levels of many of its enzymes at any one time. When the bacterium comes in contact with wood, for instance, a few of its enzymes break down some of that wood. A product of that tiny reaction is a sugar called laminaribiose that diffuses into the cell. There it deactivates a repressor for two genes, which wake up and start pumping out the two triggers the full production of wood-degrading enzymes CelC and LicA.
Wu's paper shows the first time the triggering pathway for enzyme production in this bacterium has been revealed, and it was only possible because C. thermocellum genome was just recently sequenced, thanks to Wu's collaboration with the U. S. Department of Energy. With its 100 busy enzymes, the entire genome had to be observed as a whole, since fiddling with combinations of two, three, or more enzymes at a time would have taken "more than our lifetime," Wu says.
Wu is now working to re-engineer C. thermocellum to express an abundance of particular genes so it can readily and efficiently produce ethanol from a particular biomass. He's also continuing the genome-wide search for enzyme combinations that will degrade and ferment grasses, corn stovers, and even food waste.
"I don't think this is the revolution that makes ethanol a mainstay," says Wu, "but I believe this is a part of what will lead to the revolution."
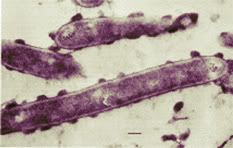
Image: The bacterium Clostridium thermocellum.
More information:
Michael Newcomb, Chun-Yu Chen, and J. H. David Wu, Induction of the celC operon of Clostridium thermocellum by laminaribiose, [*abstract], February 27, 2007 Proc. Natl. Acad. Sci. USA, 10.1073/pnas.0700087104.
 --------------
--------------


 Saab will introduce its BioPower flex-fuel options to its entire 9-3 range, including Sport Sedan, SportCombi and Convertible bodystyles, at the Geneva auto show.
Saab will introduce its BioPower flex-fuel options to its entire 9-3 range, including Sport Sedan, SportCombi and Convertible bodystyles, at the Geneva auto show.








0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home