Biohydrogen, a way to revive the 'hydrogen economy'?
 Ever since Joseph J. Romm wrote The Hype About Hydrogen, more and more scientists have spoken out against the gas as a viable carrier for energy, and attention has radically shifted to biofuels. It looks like the 'hydrogen economy' has died, at least as a scientific concept. The main drawback with H2 is that it must first be produced, requiring a primary energy source, and this is where scientists see major obstacles: when fossil fuels are used, hydrogen is no longer a clean fuel and causes greenhouse gas emissions; when environmentally friendly wind electricity is used to generate the gas, only one-quarter of the energy generated by the wind turbine is eventually used to move a car. The rest is lost during transport and energy conversion; via solar power, the final yield is even lower.
Ever since Joseph J. Romm wrote The Hype About Hydrogen, more and more scientists have spoken out against the gas as a viable carrier for energy, and attention has radically shifted to biofuels. It looks like the 'hydrogen economy' has died, at least as a scientific concept. The main drawback with H2 is that it must first be produced, requiring a primary energy source, and this is where scientists see major obstacles: when fossil fuels are used, hydrogen is no longer a clean fuel and causes greenhouse gas emissions; when environmentally friendly wind electricity is used to generate the gas, only one-quarter of the energy generated by the wind turbine is eventually used to move a car. The rest is lost during transport and energy conversion; via solar power, the final yield is even lower.There might however be a more efficient way to produce the gas, in such a way that the stages of the conversion process reinforce each other, instead of working against each other with energy losses as a result. We are talking about the production of hydrogen from biomass -- biohydrogen -- in a process that is being designed by several science institutions in a joint EU program called 'Hyvolution' that was launched at the beginning of this year.
Hyvolution fits in a futuristic vision of small-scale sustainable energy production from locally produced sources, in this case biomass. The end goal is to deliver prototypes of process modules which are needed to produce hydrogen of high quality in a bioprocess which is fed by multiple biomass feedstocks. As such, it is envisioned that small-scale modules can be used in a decentralized energy production structure, which makes them interesting for remote regions in the developing world (an ideal system for energy leapfrogging).
Bacteria do the work
But how does the biohydrogen process work? In principle it comes down to producing pure hydrogen from biomass in a non-thermal process, through the combination of a thermophilic fermentation stage with a photoheterotrophic fermentation stage. In the first fermentation thermophilic bacteria are used to start the conversion of biomass which offers two important advantages. First, thermophilic fermentation at ≥70°C is superior in terms of hydrogen yield as compared to fermentations at ambient temperatures. In thermophilic fermentations, glucose is converted to, on average, 3 moles of hydrogen and 2 moles of acetate as the main by-product. In contrast, in fermentations at ambient temperatures, the average yield is only 1 to 2 moles of hydrogen per mole of glucose and butyrate, propionate, ethanol or butanol are the main by-products. The second advantage is the production of acetate as the main by-product in the first fermentation. Acetate is a prime substrate for photoheterotrophic bacteria. Through the combination of thermophilic fermentation with photoheterotrophic fermentation, a complete conversion of the substrate to hydrogen and CO2 can be established:
 biomass :: bioenergy :: biofuels :: sustainability :: bacteria :: fermentation :: hydrogen :: biohydrogen ::
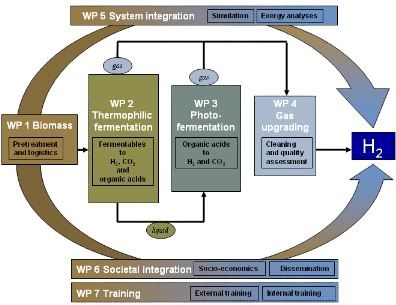
biomass :: bioenergy :: biofuels :: sustainability :: bacteria :: fermentation :: hydrogen :: biohydrogen ::The Hyvolution program is structured around this core issue with a design aimed at closely associating the events in the chain from biomass to hydrogen. The process starts with the conversion of biomass to make a suitable feedstock for the bioprocess [see picture) (work package 1 / 'WP1'). The ensuing bioprocess is optimized in terms of yield and rate of hydrogen production through integrating fundamental and technological approaches, addressed in workpackage 2 and 3. Dedicated gas upgrading is developed for high efficiency at small-scale production units dealing with fluctuating gas streams (WP4). Production costs will be reduced by system integration combining mass and energy balances (WP5). The impact of small-scale hydrogen production plants is addressed in socio-economic analyses performed in work package 6.
Ten EU countries countries collaborate on the Hyvolution program, with Turkey and Russia joining as well with prominent specialists from academia and industries. Six small and medium sized enterprises are represented as well. The participants in Hyvolution have a complementary value in being biomass suppliers, end-users or stakeholders for developing specialist enterprises and stimulating new agro-industrial development.
Hyvolution is part of the European Union's efforts on biohydrogen, which comprise several other research programs.
Disadvantages remain
However, many factors still plead against hydrogen as a viable energy carrier, even when derived from an efficient process using biomass as feedstock: after it has been produced, the gas is unstable and thus requires storage under high pressure which in itself costs energy. Biofuels are much more easy to handle and the hydrogen contained in them comes in a stable form. Speaking in terms of net efficiency, from a raw stream of biomass, roughly 40-50% can be thermochemically converted into a liquid fuel through steam reforming; the percentage is even higher for hydrogen, 50-60%, but after the compression phase, biofuels come out on top as the easiest and most efficient way to store hydrogen in a fuel ready to be used by consumers.
In short, why make the detour via hydrogen, when biomass can be turned into stable, easy to handle biofuels? Wim van Swaaij, professor of thermo-chemical conversion at Netherlands' Twente University, thinks that even the most efficient biohydrogen production process can never achieve the conversion efficiencies found in ordinary biofuel production.
Moreover, producing hydrogen, either through electrolysis using nuclear or renewable electricity, or refined from biomass or fossil fuels, requires massive amounts of water. One kilogram of hydrogen requires nine litres of water. The production of ordinary first generation biofuels is much less water intensive.
 -------------------
-------------------
 Spanish company Ferry Group is to invest €42/US$55.2 million in a project for the production of biomass fuel pellets in Bulgaria.
The 3-year project consists of establishing plantations of paulownia trees near the city of Tran. Paulownia is a fast-growing tree used for the commercial production of fuel pellets.
Spanish company Ferry Group is to invest €42/US$55.2 million in a project for the production of biomass fuel pellets in Bulgaria.
The 3-year project consists of establishing plantations of paulownia trees near the city of Tran. Paulownia is a fast-growing tree used for the commercial production of fuel pellets.









0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home